Reports
ARVO Update: Evolving Strategies for Diabetic Macular Edema
This report is based on medical evidence presented at sanctioned medical congress, from peer reviewed literature or opinion provided by a qualified healthcare practitioner. The consumption of the information contained within this report is intended for qualified Canadian healthcare practitioners only.
PHYSICIAN PERSPECTIVE - Viewpoint based on presentations from the Annual Meeting of the Association for Research in Vision and Ophthalmology (ARVO)
Seattle, Washington / May 5-9, 2013
Guest Editor:
R. Geoff Williams, MD
Southern Alberta Eye Center
Canada
Introduction
An ongoing evolution in the treatment of diabetic macular edema (DME) is being driven by an expansion of therapeutic options. At the 2013 Annual Meeting of the Association for Research in Vision and Ophthalmology (ARVO), new data provided insight about how a growing array of vascular endothelial growth factor (VEGF) inhibitors might be employed alone, alongside retinal laser photocoagulation, or in combination with other emerging therapies like steroid implants. The expanding options come at an opportune time. The rising rates of diabetes – and, in turn, the ophthalmic complications of diabetes – are expected to create a growing health burden in Canada and most other industrialized countries. The ARVO data document progress in the effort to establish the relative value of treatment choices.
Background
DME, generally characterized by retinal thickening or the presence of hard exudates,1 is one of the most common forms of retinopathy and loss of visual acuity in individuals with diabetes.2 In a retrospective analysis of medical records from southern Ontario, the incidence of DME in adults with diabetes was 15.7%,3 but both the likelihood and the severity of DME increases progressively with duration of disease.4-5
The pathogenesis of DME is complex, but upregulation of angiogenesis is implicated in a series of events that also includes cytokine release and pro-inflammatory processes.6 While hyperglycemia may contribute directly to neuronal damage that advances vision loss in DME,7 the hypoxia from impaired vascular function upregulates VEGF signaling to induce angiogenesis, which, in turn, contributes to the hyperpermeability of retinal vessels in patients with diabetes.8
In the most recent guidelines from the American Academy of Ophthalmology, retinal laser photocoagulation remains listed as a first-line therapy for DME,9 but the benefit of this therapy is limited to preserving vision rather than reversing damage.10 Newer therapies, including inhibitors of VEGF and anti-inflammatory therapies, such as steroids, may be more effective at addressing the underlying pathophysiologic processes. The first VEGF inhibitor to complete phase 3 trials in DME, ranibizumab, produced highly significant improvements in vision relative to baseline and sham injections (Figure 1).11 Regulatory approval for ranibizumab in the treatment of DME was granted by Health Canada in 2011.
At the ARVO 2013, new data expanded information about how VEGF inhibitors should be considered in the management of DME. These data are useful for considering whether VEGF inhibitors are interchangeable, where steroid implants may fit in the control of DME, whether VEGF inhibitors might be combined with laser therapy, and whether strategies can be developed to extend the duration of benefit with any of the current therapies.
New Data on Single Agent Therapies
Ranibizumab was the first agent tested in phase 3 trials and approved for the treatment of DME, and the large clinical trials program with that agent continues to generate new data from follow-up and subgroup analyses. In one presentation at the ARVO 2013, predictors of early vision gains in the RIDE and RISE trials were analyzed using the Kaplan-Meier method.12 This analysis included 759 patients.
Even though all patients were eligible for as-needed macular laser treatment by month 3 of the study, both the time to a 15-letter or better gain in best corrected visual acuity (BCVA) or a gain of 20/40 vision was significantly shorter on either dose of ranibizumab (0.3 mg or 0.5 mg) relative to sham. Late responses were common. Half of the patients who gained 15 or more letters did so after 11 months of therapy. The point at which 50% of ranibizumab patients who ever achieved a vision of 20/40 occurred in 71 days in the 0.3-mg dose group and in 58 days in the 0.5-mg dose group.
Figure 1.
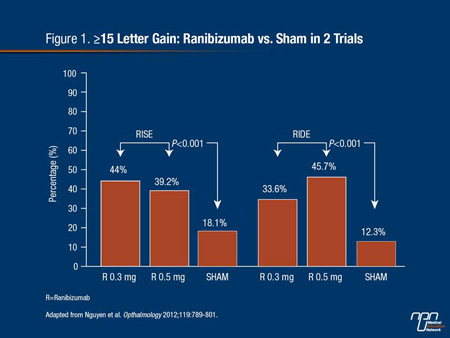
Predictors of early visual improvements, regardless of therapy, included younger age and a better BCVA at baseline. The presence of subretinal fluid predicted early vision gain in the ranibizumab group but not in the sham therapy group. Conversely, lower central foveal thickness and no prior therapy for DME predicted early vision gain in the sham group but not in the ranibizumab group.
The trials of ranibizumab in DME followed previous phase 3 studies that generated regulatory approval for the treatment of neovascular age-related macular degeneration (AMD). The subsequent attention to intravitreal injection of VEGF inhibition for these conditions has generated a still unresolved controversy regarding the ability of alternative VEGF inhibitors to provide comparable efficacy and safety. Some of the alternatives, notably bevacizumab, have never undergone rigorous safety and efficacy trials and are not formulated in ophthalmic doses. Several studies support the premise that these drugs may not be interchangeable.
In one such study at ARVO 2013, ranibizumab, bevacizumab, and aflibercept were compared for binding affinities to recombinant human VEGF (rhVEGF) under uniform conditions.13 While the study found that ranibizumab had a slower dissociation rate constant, indicating a higher affinity, relative to bevacizumab or aflibercept, the authors also emphasized that these agents behaved differently in the different assay formats used to conduct the experiments. These differences, such as the variable receptor binding characteristics observed across assays, could have implications for clinical variability in potency and pharmacokinetics. The authors indicated that the results indicate that comparable clinical efficacy cannot be assumed.
In the controversy about the relative effects of VEGF inhibitors, the debate has been most animated in regard to the substitution of bevacizumab for ranibizumab. Driven by the lower relative cost of reconstituting bevacizumab for ophthalmic use relative to the approved ranibizumab formulation, the debate has persisted in the absence of level one comparative studies. This was not resolved by data presented at ARVO 2013. Concerns about the widespread off-label use of bevacizumab involve both relative activity in the eye and relative risk of distribution of this agent – outside of the retinal space – due to risk of systemic adverse events.
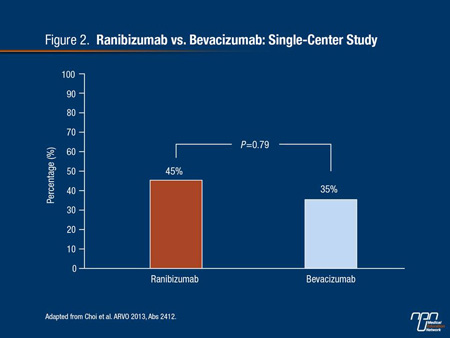
The results of the small case-control and retrospective studies that have been done, including one presented at ARVO 2013,14 often show unequal results, encouraging the conclusion that these agents are not interchangeable. In the ARVO study of 32 patients with DME, 45% of ranibizumab versus 35% of bevacizumab patients had gained at least 3 lines of visual acuity by 6 months (Figure 2), but the results were difficult to interpret because of differences in baseline characteristics and number of injections. Appropriately, the authors acknowledged the need for an adequately powered head-to-head comparison.
Figure 2.
In another study that included both ranibizumab and bevacizumab, the goal was to determine whether ranibizumab could rescue DME patients with a poor response to bevacizumab.15 In this prospective but non-randomized study, 43 patients with a poor response to at least 2 injections of bevacizumab were treated with 3 monthly injections of 2.0 mg of ranibizumab.
Mean visual acuity, which was 59 letters at baseline, improved by 6.4 letters at month 3 and 8.8 letters by month 6. In addition, there was a decrease in subfield thickness by 114 μm at 3 months and 165 μm at 6 months. While the authors concluded that an incomplete or poor response to bevacizumab in DME patients should not be a contraindication to considering ranibizumab, the efficacy of one VEGF inhibitor after failure of a prior VEGF inhibitor lends support to the premise that these agents may not be interchangeable.
In regard to steroid implants, the MOZART study provided the most significant new data at ARVO 2013.16 In this multicenter, non-comparative evaluation, the efficacy and safety of the intravitreal dexamethasone implant marketed under the name Ozurdex was evaluated in 59 patients (69 eyes). The minimum follow-up was 6 months and the mean follow-up was 9.8 months. The mean age of the patients was 65 years, and the average duration of diabetes at the time of implant was 15 years. Only 24% were naive to any previous therapy for DME.
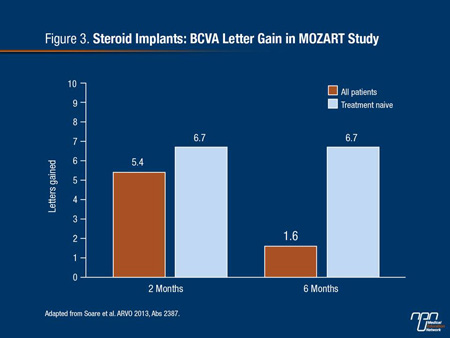
Over a follow-up of 6 months, a gain greater than 15 letters was observed in 28% of the patients and a loss of greater than 15 letters was observed in 6% of patients. The BCVA letter score improvement from a mean score of 54.4 letters at baseline was 5.4 letters at month 2 and 1.6 letters and month 6. However, greater response was achieved in treatment naive patients with a 6.7 letter score improvement at month 2 and 6.7 letter score improvement at month 6 (Figure 3). Ocular hypertension of greater than 25 mmHg was observed in 7% of patients. Other adverse events included progression to cataract in 4% and vitreous hemorrhage in 1.5%. The authors characterized the safety profile as favourable.
Figure 3.
New Data on Combining Therapies in DME
VEGF inhibitors are widely considered a major advance in the treatment of DME, but combination treatments are being pursued when response is inadequate. Of combination studies presented at ARVO 2013, the most rigorous was the RELATION trial, which was double-masked. All 128 participants received laser photocoagulation and were randomized in a 2:1 ratio to ranibizumab or sham injections given monthly for 4 months and then as needed.17 The follow-up was 12 months.
The BCVA letter gain was 6.5 letters on the combination versus 1.4 letters (P=0.001) on laser therapy plus sham injections. This was consistent with a significantly higher reduction in the total retinal volume in the combination group. Of those with proliferative diabetic retinopathy at baseline, 40% in the ranibizumab group and none in the sham group had regression.
In another study, the goal was to evaluate whether prompt or deferred laser treatment provides better outcomes after initiation of ranibizumab.18 At 3 years, the percentages of eyes with central subfield thickness of 250 μm or greater was the same (36%). Even though patients in the deferred group ultimately received more injections (15 vs. 12; P=0.007), 54% of those in the deferred group never received laser treatment.
In another therapeutic approach for which data were presented at ARVO 2013, the value of peripheral photocoagulation after a single injection of bevacizumab was evaluated for prevention of DME.19 In this study, 41 patients were randomized to receive an intravitreal injection of 1.25 mg of bevacizumab alone or the same injection followed by peripheral photocoagulation for ischemic retina area as determined by fluorescein angiography. At 5 months relative to baseline, there was significant BCVA improvement (P<0.05) on the combination, but not in the group receiving bevacizumab alone.
Summary
The proliferation of therapies has permitted a variety of novel strategies to be explored in the effort to control DME. Within these strategies, VEGF inhibitors are likely to play an increasingly important therapeutic role due to their ability to address underlying pathophysiologic processes. Comparisons will be important for understanding potential differences between VEGF inhibitors. Due to the significant potential risks from systemic exposure, safety data may be at least as important as efficacy data. At ARVO 2013, a pooled safety data analysis from 22 randomized studies, including 5 conducted in DME, provided reassurance of the safety of ranibizumab,20 but it is not clear that these findings can be extended to other VEGF inhibitors due to differences in dose, formulation, and drug characteristics. Such reassurance is particularly important in the context of diabetes, which imposes its own adverse effects on vascular function. Due to the progressive and persistent threat of vision loss in DME, both the safety and efficacy of extended therapy is needed for all of the available treatment options.
References
1. Romero-Aroca P. Targeting the pathophysiology of diabetic macular edema. Diabetes Care 2010;33(11):2484-5.
2. Bandello F, Battaglia Parodi M, Lanzetta P, et al. Diabetic macular edema. Dev Ophthalmol 2010;47:73-110.
3. Petrella RJ, Blouin J, Davies B, Barbeau M. Prevalence, Demographics, and Treatment Characteristics of Visual Impairment due to Diabetic Macular Edema in a Representative Canadian Cohort. J Ophthalmol 2012;2012:159167.
4. Chen E, Looman M, Laouri M, et al. Burden of illness of diabetic macular edema: literature review. Curr Med Res Opin 2010;26(7):1587-97.
5. Klein R, Klein BE, Moss SE, Cruickshanks KJ. The Wisconsin Epidemiologic Study of Diabetic Retinopathy: XVII. The 14-year incidence and progression of diabetic retinopathy and associated risk factors in type 1 diabetes. Ophthalmology 1998;105(10):1801-15.
6. Bhagat N, Grigorian RA, Tutela A, Zarbin MA. Diabetic macular edema: pathogenesis and treatment. Surv Ophthalmol 2009;54(1):1-32.
7. Barile GR, Pachydaki SI, Tari SR, et al. The RAGE axis in early diabetic retinopathy. Invest Ophthalmol Vis Sci 2005;46(8):2916-24.
8. Murata T, Ishibashi T, Khalil A, Hata Y, Yoshikawa H, Inomata H. Vascular endothelial growth factor plays a role in hyperpermeability of diabetic retinal vessels. Ophthalmic Res 1995;27(1):48-52.
9. AAO Retina Pane: Preferred Practice Pattern Guidelines for Diabetic Retinopathy. 2012. (Accessed at www.aao.org/ppp.)
10. Treatment techniques and clinical guidelines for photocoagulation of diabetic macular edema. Early Treatment Diabetic Retinopathy Study Report Number 2. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology 1987;94(7):761-74.
11. Nguyen QD, Brown DM, Marcus DM, et al. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology 2012;119(4):789-801.
12. Morse LS, Yau L, Tuomi L. Clinically significant improvement in visual acuity and predictors of early vision gains in the RIDE and RISE phase III trials of ranibizumab for diabetic macular edema. In: Association for Research in Vision and Ophthalmology (ARVO); 2013; Seattle, Washington: Abs. 1241; 2013.
13. Wang X, Yang J. Analysis of the binding affinity of vascular endothelial growth factor A (VEGF) ranibizumab, aflibercept, and bevacizumab. In: Association for Research in Vision and Ophthalmology (ARVO); 2013; Seattle, Washington: Abs. 1961; 2013.
14. Choi D, Lim JI. Intravitreal bevacizumab and ranibizumab for diabetic macular edema; a single center retrospective study. In: Association for Research in Vision and Ophthalmology; 2013; Seattle, Washington: Abs. 2412; 2013.
15. Pieramici DJ, Nasir M, Castellarin A. Ranibizumab 0.5 mg and 2.0 mg to treat diabetic macular edema in patients with poor response to bevacizumab. In: Association for Research in Vision and Ophthalmology (ARVO); 2013; Seattle, Washington: Abs. 2413; 2013.
16. Soare S, Hajjar C, Parrat E. Multicenter Ozurdex assessment for diabetic macular edema (MOZART) study. In: Association for Research in Vision and Ophthalmology; 2013; Seattle, Washington: Abs. 2387; 2013.
17. Lohmann CP, Voegeler J, Liakopoulos S. Double-masked trial demonstrates superiority of combined ranibizumab plus laser versus laser in patients with diabetic macular edema with or without proliferative diabetic retinopathy. In: Association for Research in Vision and Ophthalmology (ARVO); 2013; Seattle, Washington: Abs. 1239; 2013.
18. Rauser ME. Intravitreal ranibizumab for diabetic macular edema with prompt vs deferred laser treatment: 3-year randomized trial results. In: Association for Reserch in Vision and Ophthalmology (ARVO); 2013; Seattle, Washington: Abs. 212; 2013.
19. Takamura Y, Tomomatsu T, Matsumura T. Photocoagulation for peripheral nonperfusion areas to prevent the recurrence of diabetic macular edema after single intravitreal injection of bevacizumab. In: Association for Research in Vision and Ophthalmology; 2013; Seattle, Washington: Abs. 2377; 2013.
20. Avery RL, Francom SF, Lai P. Meta-analysis examining the systemic safety profile of intravitreal ranibizumab injections in AMD, RVO, and DME. In: Association for Research in Vision and Ophthalmology (ARVO); 2013; Seattle, Washington: Abs. 1535; 2013.