Reports
Bone-targeted Therapies for Patients with Advanced Prostate Cancer
This report is based on medical evidence presented at sanctioned medical congress, from peer reviewed literature or opinion provided by a qualified healthcare practitioner. The consumption of the information contained within this report is intended for qualified Canadian healthcare practitioners only.
PRIORITY PRESS - 107th Annual Meeting of the American Urological Association
Atlanta, Georgia / May 19-24, 2012
Atlanta - Most patients with advanced prostate cancer will experience at least one skeletal-related event (SRE) without bone-directed therapy. In men with castration-resistant disease, an intravenous bisphosphonate and an anti-RANK ligand monoclonal antibody (MAb) have both demonstrated efficacy in preventing or delaying SREs. These agents, which inhibit bone resorption by different mechanisms, do not appear to be interchangeable. In a major trial discussed here at the AUA, the MAb was associated with a modest advantage in regard to bone-related events but a higher risk of serious adverse events.
Chief Medical Editor: Dr. Léna Coïc, Montréal, Quebec
To prevent skeletal-related events (SREs) in patients with castration-resistant prostate cancer, both zoledronic acid and denosumab prevent bone resorption by blocking the activity of osteoclasts. The bisphosphonate zoledronic acid inhibits osteoclasts along several different pathways including an upregulation of apoptosis. The monoclonal antibody (MAb) denosumab binds to a receptor involved in osteoclast maturation. In a trial designed to compare the 2 agents, the MAb was more effective for delaying SREs (20.7 vs. 17. months; HR 0.82; P=0.008) but was associated with a greater risk of serious adverse events (SAEs) overall and of hypocalcemia specifically (13% vs. 6%; P<0.0001) (Lancet 2011;377:813-22). New data presented here at the AUA attempted to put these differences into clinical context.
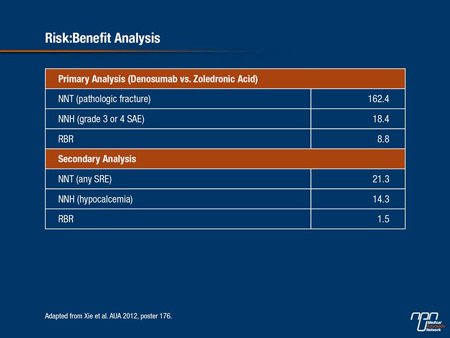
Our objective was to conduct a risk:benefit analysis based on the number needed to treat [NNT] and the number needed to harm [NNH] for denosumab and zoledronic acid,” reported Dr. Jipan Xie, Analysis Group, New York, New York.
In this study, data from the comparative trial were reapplied to calculate the NNT for having a first on-treatment bone fracture and the NNH for an SAE (grade 3 or 4) with either agent compared to the other. The NNT and NNH were then used to determine the risk:benefit ratio (RBR). As a secondary end point, the same analyses were performed using SREs as the efficacy outcome and hypocalcemia as the AE outcome. All analyses assumed that patients discontinued treatment after the first on-treatment SRE.
Risk:Benefit Analysis
On the basis of the phase III head-to-head comparison, the NNT for preventing one fracture by using denosumab rather than zoledronic acid is 162.4. The NNH for incurring a grade 3 or 4 SAE is 18.37. This means that although more than 162 patients would need to be treated with the monoclonal antibody rather than zoledronic acid to prevent a fracture, only 18.4 patients would need to be treated to incur an additional grade 3 or 4 SAE. The RBR is 8.8. As Dr. Xie pointed out, the RBR of 8.8 “indicates that 8.8 times more patients would need to be treated to prevent a pathological fracture as the first SRE than to have one additional grade 3 or 4 SAE.”
A secondary analysis that altered the variables produced a similar result. In this calculation the NNT was determined for any SRE not just pathological fracture and the NNH was determined for hypocalcemia. In this analysis, the NNT for the first SRE for using denosumab rather than zoledronic acid was 21.3 while the NNH was 14.3, producing a RBR of 1.5. Again, this RBR indicates a higher risk of having an AE than a SRE benefit by using denosumab.
Dr. Xie reported, “More patients treated with denosumab instead of zoledronic acid would experience the undesirable outcomes of SAEs or hypocalcemia than the desirable outcomes of avoiding a first on-treatment pathologic factor or SRE.”
Overall, estimates produced by the NNH analyses showed that 71.9% of MAb patients would be expected to have a grade 3-4 AE and 12.83% would develop hypocalcemia. Estimates for the bisphosphonate were 66.46% and 5.82%, respectively. Comparison of the 2 treatment arms resulted in an absolute risk reduction of 5.44% for grade 3-4 AE and 7.01% for hypocalcemia in favour of the bisphosphonate. When these results were combined, Dr. Xie calculated that >8 patients treated with denosumab rather than zoledronic acid would develop an SAE for each pathologic fracture prevented. The data predict that 11 patients would develop hypocalcemia
for each pathologic fracture prevented. Relative to any bonerelated event, there would be one case of SAE and 1.5 cases of hypocalcemia predicted for each SRE prevented with the MAb.
“The high risk:benefit ratio suggests that despite the better efficacy of denosumab, its benefits may not outweigh its added risks,” Dr. Xie remarked.
Table 1.
Delaying Biochemical Progression
When the treatment goal is a delay of biochemical recurrence of metastatic prostate cancer, a multicentre, prospective phase II clinical trial has suggested that combining zoledronic acid with androgen deprivation therapy (ADT) might be effective relative to ADT alone.
The findings emerged from an evaluation of 54 men who had a median age of 72 and median baseline prostate-specific antigen (PSA) values of 249 ng/mL. They received combined ADT with bicalutamide and goserelin, plus intravenous zoledronic acid 4 mg q4 weeks. Investigators assessed markers of bone turnover (BAP, I-CTP and NTx) at baseline and then every 3 months.
Outcomes in the patient population were compared with those of a historical group of 161 men with metastatic prostate cancer treated with ADT alone, according to Dr. Hirotsugu Uemura, Kinki University Faculty of Medicine, Sakai, Japan.
Baseline values of NTx ≥100 nmol/mmol, PSA >100 ng/mL and number of involved organs (≥2) were associated with a shorter time to biochemical relapse. Treated patients who received zoledronic acid had a median follow-up of 31 months, and a third of patients completed 24 months of treatment with the bisphosphonate.
Median progression-free survival was 24 months in the cohort that received zoledronic acid compared with 15 months in the historical controls (P=0.0439). Median overall survival had not been reached in the bisphosphonate cohort vs. a median of 65 months in the historical controls (P=0.0060). AEs included osteonecrosis of the jaw in 3.8% of patients and renal dysfunction in 5.6% of patients treated with zoledronic acid.
Investigators concluded that combination therapy of 2-year zoledronic acid treatment and ADT might have better efficacy than ADT alone in patients with treatment-naive metastatic prostate cancer and could be considered particularly for patients whose baseline urine NTx is below 100 nmol/mmol.
Findings from a National Cancer Database
An analysis of data from the U.S. SEER (Surveillance, Epidemiology and End Result) database has provided evidence that zoledronic acid, which has been used at an increasing rate in patients with metastatic prostate cancer over a recent 5-year period, is associated with a decreased fracture risk. In this study, a cohort of 992 individuals was derived from 8028 metastatic cancer patients after a variety
of exclusions, including those for radiotherapy or a previous diagnosis of osteopenia. Hazard ratios (HR) were derived from a Cox proportional model for time-to-fracture analysis.
“We determine that increasing age [HR 2.7; P=0.04 for those 80 to 84 years] and previous radiotherapy [HR 2.8; P=0.03] were associated with increased risk of bone fractures. In contrast, receipt of zoledronic acid was associated with decreased risk of bone fracture over time [HR 0.43; P=0.003],” reported Dr. Matthew J. O’Shaughnessy, University of Minnesota, Minneapolis, told delegates. The study also revealed that increased zoledronic acid use was associated with a more recent year of diagnosis and age <80 years. However, there was no significant variation in zoledronic acid use across race, income, education, Charlson comorbidity score, tumour stage or grade.
Overall, based on the growing use of zoledronic acid that correlates with a falling rate of bone fracture, Dr. O’Shaughnessy concluded that “continued use of agents to reduce fracture risk is supported in this population.”
Treatment Effect on WNT Signalling
Better understanding of bone-targeted therapies’ mechanistic effects may help optimize use of these agents. To that end, investigators here at the AUA reported that WNT signalling was significantly altered by zoledronic acid. The findings emerged from an analysis of tumour specimens from men with localized and advanced prostate cancer, as well as control specimens from men with benign prostate hyperplasia. Investigators compared gene expression patterns between types of prostate cancer and the effect of zoledronic acid on expression, according to Dr. Kati Erdmann, Technical University of Dresden, Germany.
The results confirmed a suspected association between WNT signalling and prostate cancer progression, as WNT was upregulated in advanced cancers. Moreover, researchers found that WNT was differentially expressed by osteotropic vs. non-osteotropic prostate cancer cells. Exposure to the bisphosphonate resulted in downregulation of WNT mRNA.
Summary
A risk:benefit calculation from a randomized clinical trial showed that compared with zoledronic acid, denosumab use reduced risk of SRE in men with castration-resistant prostate cancer but increased risk of adverse events. These findings have placed these disparate benefits and risks into perspective. On an ITT basis, the increased risk of SAEs was greater than the relative protection from bone events, indicating that the balance appears to favour the bisphosphonate. Data presented here at the AUA also indicated that zoledronic acid might delay the time to biochemical progression when added to ADT for men with advanced prostate cancer and bone metastases. An analysis of community experience suggests that increasing the use of prophylactic therapies has been associated with a decreased risk of pathologic fracture in patients with advanced disease.