Reports
Changing Landscape in the Treatment of Axial Disease in Patients with Axial PsA and axSpA
This report is based on medical evidence presented at sanctioned medical congress, from peer reviewed literature or opinion provided by a qualified healthcare practitioner. The consumption of the information contained within this report is intended for qualified Canadian healthcare practitioners only.
MEDICAL FRONTIERS - EULAR European Congress of Rheumatology
Madrid, Spain / June 12-15, 2019
Madrid – Axial manifestations are present in a high proportion (25%-75%) of patients with psoriatic arthritis (PsA). NSAIDs continue to be the mainstay of treatment in the early phases of axial PsA and axial spondyloarthritis (axSpA). When NSAIDs fail, several therapies are available and ongoing research may clarify how best to employ these new options. Results from the primary analysis of the MAXIMISE trial in patients with axial PsA, highlighted here at EULAR, showed that IL17A inhibition rapidly led to improvement in response compared with placebo. An emerging consensus also suggests that early treatment with biologic disease-modifying anti-rheumatic drugs (bDMARDs), before irreversible damage has occurred, can optimize outcomes.
Chief Medical Editor: Dr. Léna Coïc, Montréal, Quebec
“Enthesitis and inflammation are central pathophysiologic steps in ankylosing spondyloarthritis (AS),” explained Professor Georg Schett, Department of Internal Medicine 3, Friedrich-Alexander University, Erlangen, Germany, during the presentations here this week. Although inflammation is seen in areas of high mechanical stress, in the context of inflammatory back pain, the IL17 pathway plays a key role in the pathological process and inflammation responsible for damage and progression. According to Dr. Ellen Gravallese, Division of Rheumatology, University of Massachusetts Medical School, USA, “In my opinion, if you can control inflammation, you can control entheseal bone formation, and you can do this by blocking IL17A early in the process.” IL17 is therefore considered a primary therapeutic target for the treatment of axial SpA and patients with axial manifestations in PsA.
Support for the efficacy of the anti-IL17 strategy targeting enthesitis in the case of patients with AS was provided by a pooled post-hoc analysis of 693 patients with baseline enthesitis (Maastricht AS Enthesitis Score [MASES] >0) enrolled in the 4 MEASURE studies presented by Prof. Schett. This analysis demonstrated secukinumab 150 and 300 mg were associated with higher reductions in enthesitis according to overall MASES and axial entheseal site assessments compared to placebo at Week 16, with continued reductions through 52 weeks.
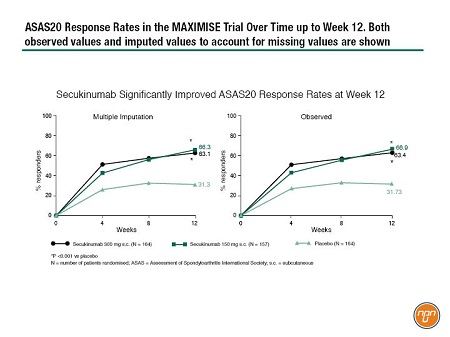
In the case of patients with axial PsA, the MAXIMISE study was designed to test the utility of IL17 inhibition. This ongoing study is a randomized, double-blind, phase III study in which 503 patients with PsA, and active spinal disease and spinal pain with an inadequate response to at least 2 NSAIDs over a 4-week period, were randomized 1:1:1 to secukinumab 300 mg, secukinumab 150 mg, or placebo (Figure 1). After 12 weeks, patients assigned to active treatment continued at the same dose while placebo patients were randomized to either secukinumab 300 mg or secukinumab 150 mg for a further 40 weeks. The primary efficacy outcome measure was Assessment of Spondyloarthritis International Society (ASAS) 20 response at Week 12. The Week 12 results are now available and were presented by Dr. Xenofon Baraliakos, Rheumatology Center, Ruhr-University Bochum, Germany, at this year’s EULAR congress. The treatment groups were well balanced in terms of demographics and history of axial disease. Approximately two-thirds of the patients in the active treatment groups had responded at Week 12 while approximately one-third of placebo-treated patients were responders. “In terms of dynamics of treatment response, we see a fast response that increased slightly over time,” explained Dr. Baraliakos (Figure 2). Response was observed regardless of concomitant methotrexate use. The safety data were entirely consistent with previous reports.
Figure 1. Overview of the MAXIMISE Trial Design
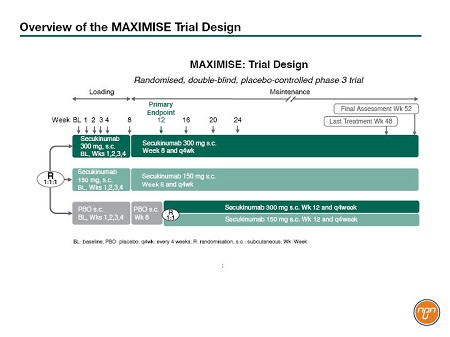
Figure 2. ASAS20 Response Rates in the MAXIMISE Trial Over Time up to Week 12. Both observed values and imputed values to account for missing values are shown
What Biologic Agents Should be Used in Patients with Inflammatory Back Pain?
Current guidelines recommend NSAIDs in a first instance. When patients fail treatment with at least 2 of these agents, the next step is to switch to a biological agent. Often, this will be an anti-TNF agent, partly because this class is more established, however, the presence of comorbidities and other domains may also be a consideration. In particular, the efficacy of anti-IL17 agents for managing skin manifestations in patients with axial PsA may suggest that these agents would be preferable in patients with axial disease and skin involvement. A late breaking abstract presented the results of an open label head-to-head trial (SPIRIT) of an IL17 inhibitor (ixekizumab) and a TNF inhibitor (adalimumab) in biologic-naïve patients with active PsA and psoriasis. The IL17 inhibitor was found to be superior to the TNF inhibitor for the composite primary endpoint ACR50 and PASI100 response at 24 weeks. Further head-to-head data will be available from the EXCEED trial comparing secukinumab with adalimumab in patients with active PsA (completion expected later this year).
A more complex question is which biologic agent should be used after failure of a first or subsequent biologic agent. The pivotal trials for available biologic agents recruited mainly biologic-naïve patients. The MEASURE-2 trial did also recruit anti-TNF experienced patients, and although a response to secukinumab was observed, it was lower than in their biologic-naïve counterparts. In general though, the evidence to support decisions once a first-line biologic has failed is largely derived from real world data. Although real world evidence has, in the past, often been dismissed as weak evidence on which to base decisions, there is increasing recognition that such data can provide valuable insight and help answer questions that cannot be addressed by tightly controlled clinical trials.
Real World Evidence
One of the largest registries of biologic use in SpA patients is the collaboration of registries (EuroSPA), covering 15 European countries. A study using 12 of the 15 registries in the collaboration network has investigated outcomes after first-, second- and third-line TNF inhibitors in axSpA patients. For the 7953 and 2782 patients starting their 2nd and 3rd TNF inhibitor, respectively, inactive disease according to the Ankylosing Spondylitis Disease Activity Score was reported in 18% and 13% at 6 months. “Remission rates decrease with the number of previous TNF-inhibitor therapies,” explained Prof. Mikkel Østergaard, University of Copenhagen, Denmark. Given these low and decreasing response rates following additional lines of anti-TNF agent, further research would be warranted on whether a switch to another mechanism, such as anti-IL17, would be a more appropriate strategy after failure of initial anti-TNF therapy.
Early Treatment in Non-Radiographic SpA
Across the spectrum of SpA, there is an increasing preference for early aggressive treatment with bDMARDs to limit irreversible damage due to inflammatory processes. One point for debate is management of patients with axial manifestations who do not meet the traditional criteria for AS, but would meet the criteria for non-radiographic (nr) axSpA according to the current terminology. There is a classification system for AS but no diagnostic test. “Thus, you need to rely on your expertise when treating these patients,” explained Dr. Robert Landewé, Amsterdam University Medical Center, The Netherlands.
Many patients who would not be classified as AS, without radiographic evidence of damage, do, however, show evidence of inflammation on MRI. “We need to diagnose these patients far earlier in the disease, before irreversible bony changes have occurred,” said Dr. Landewé. “The traditional AS patient does not occur particularly frequently, but if you then look for patients at earlier stages of the disease, you will find far more patients,” he added. Analysis of the German Spondyloarthritis Inception Cohort found that 12% of patients identified with nr-axSpA progressed to AS after 2 years, so the challenge is identifying which patients with nr-axSpA will progress to AS to avoid unnecessary exposure to treatment of patients not at risk. Inflammation on MRI seems to be a good predictor of those at risk. According to one study of undiagnosed patients with early inflammatory back pain (less than 2 years duration), those with severe sacroiliitis and HLA-B27 positivity had a likelihood ratio of 8.0 for developing AS whereas those with mild or no sacroiliitis, regardless of HLA-B27 status, had a likelihood ratio of 0.4. “We know that inflammation is a key trigger for progression and structural damage, and if you control inflammation, there will be less structural damage,” indicated Prof. Schett.
Guidelines
The treatment of axSpA is based on the concept of treating patients until they achieve clinical remission/inactive disease and where “clinical remission/inactive disease is defined as absence of clinical and laboratory evidence of significant disease activity, and this is an important point,” according to Prof. Denis Poddubnyy, Charité University Hospital, Berlin, Germany, “because before the focus was clinical, but now we also look at what CRP is doing, as this is a predictor of structural damage later on.” In some patients, inflammatory processes can be suppressed temporarily through NSAIDs, but this is insufficient in some patients, and an anti-TNF agent or an IL17A inhibitor is required. In the case of anti-TNF agents, early intervention has been shown to be important. For example, a post-hoc analysis of disease outcomes in patients stratified by nr-axSpA symptom duration (<5 versus ≥5 years at baseline) demonstrated that patients with shorter disease symptom duration showed greater improvements across multiple signs and symptoms of disease. Once patients have progressed, however, anti-TNF therapy does not significantly slow progression.
Conclusion
An increasing number of treatment options are now available for patients with axial PsA and those with axSpA, whether in isolation or within PsA. Data are emerging to help guide treatment decisions, which ideally should be made in a multidisciplinary setting, particularly if extra-articular manifestations are present, and in full consultation with the patient. There is a tendency to escalate earlier to biologic agents, such as IL17A inhibitors that specifically target the type of inflammation seen in axial disease, to limit progression of structural damage.
Questions and Answers
Questions and answers with Dr. Xenofon Baraliakos, Rheumatology Center, Ruhr-University Bochum, Germany and Prof. Dennis McGonagle, Faculty of Medicine, University of Leeds, UK.
Q: Is there a patient population that is not currently addressed by the guidelines or by clinical research studies?
Dr. Xenofon Baraliakos: There is a subpopulation of the disease, patients who may suffer mainly from psoriatic arthritis but who also present with increasing intensity of inflammatory back pain. Since we know that psoriatic arthritis is mainly driven by enthesitis, maybe a drug that is specific to enthesitis should be used, such as for example, secukinumab, or IL17 inhibition in general. We don’t have the full data yet, this conference is the first time we present data for these patients who responded extremely well within just 12 weeks.
Q: This is the MAXIMISE study? As this has only been presented at this congress, it will not be included in the
(updated EULAR) guidelines?
Dr. Xenofon Baraliakos : Well, I don’t think it will be included in the guidelines because from an organizational point of view, the guidelines are currently undergoing revision. But the study will certainly be taken into account in clinical practice because the axial symptoms are so important.
Q: So for axial symptoms, TNF blockers aren’t considered?
Dr. Xenofon Baraliakos : Well, they are certainly being considered, but there are currently no data to clearly show what happens with them. I don’t think they won’t work, the question is, will they work the same. If enthesitis is the main problem, IL17 inhibitors seem to be superior. The question is what is more specific for this type of patient, and from what we understand, IL17 inhibitors are perhaps the more specific agent.
Q: Is there a patient population that is not currently addressed by the guidelines or by clinical research studies?
Prof. Dennis McGonagle: Among others, there is the group of polyenthesitis patients who are difficult to recognize clinically and, also, it is difficult to use imaging unequivocally to get a definitive diagnosis. And this group is not currently recognized very well in guidelines and this is down to the fear that many patients with chronic widespread pain and mechanical pains could be misdiagnosed as the polyenthesitis group and be inappropriately treated, thus endangering patients and substantially escalating costs to providers.
Q: Is there research that would then feed into the guidelines to address these gaps?
Prof. Dennis McGonagle: There is a widespread recognition that imaging needs to be developed more to capture the heterogeneity of psoriatic arthritis. If you think of somebody with rheumatoid arthritis, you can image a joint, it is usually peripheral, with ultrasound, you can see the greyscale thickening of synovitis, you know that histologically, that represents disease. If that is left untouched longer without treatment, that will lead to erosion and destruction. You have the synovitis component in psoriatic arthritis, and you can image it in a similar way, but you can also have a large burden of axial disease and entheseal pain. Because the entheses are relatively avascular but highly innervated, you reach this situation where the patient may have lots of pain that is due to bona fide inflammation, but you are unable to visualize it on imaging. MRI is required because of the deep location and inaccessibility of the structures.
Based on scientific presentations officially recognized by the Annual European Congress of Rheumatology (EULAR) in Madrid, Spain,
June 12-15, 2019.