Reports
Expansion of MS Therapies: Multicentre Phase III Trials of First-Line Oral Agents
This report is based on medical evidence presented at sanctioned medical congress, from peer reviewed literature or opinion provided by a qualified healthcare practitioner. The consumption of the information contained within this report is intended for qualified Canadian healthcare practitioners only.
MEDICAL FRONTIERS - 28th Congress of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS)
Lyon, France / October 10-13, 2012
Lyon - Oral therapies for the control of multiple sclerosis (MS) are poised to proliferate, based on data presented at the 2012 ECTRIMS. Phase III trials have associated several agents with high rates of efficacy both in regard to the risk of relapse and to protection from disability progression. The mechanisms of the emerging oral agents differ from each other and from the only oral agent currently available in Canada. These differences may have implications for greater individualization of therapy based on response, adverse events or both. Moreover, the specific activities of these agents may create new approaches to understanding key molecular events in MS progression. In particular, some of the agents have yielded a degree of protection against disability not predicted by their protection against relapse, creating attention to the potential of mechanisms not limited to the downregulation of the inflammatory response.
New or updated phase III trial data were presented at the 2012 ECTRIMS on the oral agents, dimethyl fumarate (BG-12), laquinimod and teriflunomide. Although all of these agents have demonstrated high degrees of activity in placebo-controlled trials, each has a different mechanism of action relative to the others and to fingolimod, the only oral multiple sclerosis (MS) agent currently available in Canada. The favourable phase III results suggest these agents are likely to reach clinical practice and may provide new opportunities to individualize treatment both in relationship to disease control and to risk of adverse events (AEs).
In addition to oral administration, an intriguing potential advantage of several of the emerging agents is evidence of neuroprotection independent of anti-inflammatory activity. In summarizing the highlights of new treatment data during the closing session of ECTRIMS, Prof. Gavin Giovannoni, Barts and The London School of Medicine and Dentistry, UK, noted that the degree of protection against disability has been greater than that predicted by the protection against relapse with some of the newer oral agents as well as several injectable monoclonal antibodies now in late stages of clinical testing.
Controlled trials are needed to confirm differences in preventing disability, but each oral agent has unique mechanisms of action with potential relevance to disparate clinical activity. Of the 3 new oral agents with phase III data, BG-12 is known to activate the nuclear factor (erythroid-derived 2)-like 2 (Nrf2) pathway, which is an antioxidant response pathway implicated in cytoprotection and in the modulation of immune cell responses. Laquinimod is known to upregulate brain-derived neurotrophic factor (BDNF), which may also be neuroprotective, as well as down regulate pro-inflammatory cytokines. Teriflunomide inhibits the mitochondrial enzyme dihydro-orotate dehydrogenase, which is most closely associated with downregulation of immune mediators, but this mechanism is substantially different from existing drugs.
Based on efficacy and safety data presented or updated at ECTRIMS, each agent has a strong potential for eventual regulatory approval worldwide. Teriflunomide has already been licensed in the US. Overall, the data from controlled trials suggest a degree of efficacy on common outcomes, such as the annualized relapse rate (ARR), to be at least similar to that of the injectable disease-modifying therapies. Although the types of AEs have varied between the newer oral agents, the rates of serious AEs have been uniformly low in follow-up to date.
Updated Analysis of Phase III Trial Data
Of several sets of data presented on BG-12 at ECTRIMS in oral or poster form, the largest involved an integrated analysis of the 2 phase III trials conducted with this agent. These trials, CONFIRM (Fox et al. N Engl J Med 2012;367:1087-95) and DEFINE (Gold et al. N Engl J Med 2012;367:1098-107) were published just weeks before ECTRIMS. In the similarly designed international studies, each enlisting more than 1200 patients, relapsing-remitting MS patients were randomized to BG-12 240 mg b.i.d., t.i.d. or placebo. In CONFIRM but not DEFINE, glatiramer acetate (GA) served as a reference comparator.
In CONFIRM, the primary end point of ARR was reduced by 44% and 51% relative to placebo in the BG-12 b.i.d. and t.i.d. arms, respectively (both P<0.001) and 29% (P<0.01) in the GA arm. In DEFINE, the primary end point was proportion of patients relapse-free at 2 years. This proportion was 54% in the placebo arm, 73% and 74% in the BG-12 b.i.d. and t.i.d. arms (P<0.001 for either dose relative to placebo). BG-12 also provided protection against disease activity as assessed with numerous secondary end points. For example, the DEFINE trial associated both BG-12 doses with significant protection against disability (P=0.005 and P=0.01 for the b.i.d. and t.i.d. regimens, respectively), gadolinium-enhancing (Gd+) lesions (P<0.001 for both doses) and enlarging T2-weighted hypointense lesions (P<0.001 for both doses).
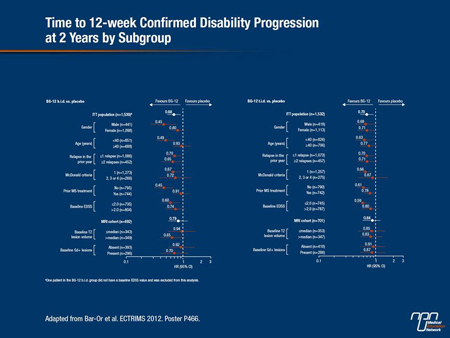
In the integrated analysis of the 2 datasets that was presented at ECTRIMS by Dr. Amit Bar-Or, Montreal Neurological Institute, McGill University, Quebec, the goal was to evaluate clinical effects in a broad array of subgroups, such as those defined by age, gender, baseline Expanded Disability Status Scale (EDSS), baseline T2 lesion volume and baseline Gd+ lesion count.
Compared to placebo, BG-12 was favoured in the integrated analysis for both primary end points across subgroup stratifications. In most cases, the relative advantage of BG-12 was statistically significant on an intention-to-treat (ITT) analysis. In addition, it “also demonstrated broadly consistent reductions in disability progression across the subgroups,” Dr. Bar-Or reported (Figure 1).
“These findings, mirroring the results reported for the overall individual study populations suggest that treatment with BG-12 is consistently effective across a broad range of clinically relevant patient subgroups,” reported Dr. Bar-Or, who suggested that the findings are consistent with the conclusion that this agent has the “potential to become a valuable treatment option.”
Figure 1.
Potential Importance of Neuroprotection
New data with laquinimod presented at the 2012 ECTRIMS was largely focused on exploring the specific potential of this agent to provide neuroprotection. Even though the ability of this agent to prevent relapse has been disappointing relative to many of the newer and existing MS therapies, neuroprotection may be particularly important for attenuating disability, as provided by laquinimod at rates as good or better than other disease-modifying agents. In the ALLEGRO trial (Comi et al. N Engl J Med 2012;366:1000-9), the authors characterized the reduction in ARR, which was the primary end point, as “modest.” Specifically, the primary end point of risk reduction for this outcome was only 33% relative to placebo (P=0.002), a protection substantially lower than that observed in randomized trials with many other active agents. For example, this is approximately 35% lower than the ARR associated with BG-12 in the CONFIRM study.
However, the 49% (P=0.002) reduction in the risk of disability confirmed at 6 months was higher than that commonly reported with other active therapies. The same disparity between protection against relapse and protection against disability was observed in the as-yet unpublished placebo-controlled phase III BRAVO laquinimod trial (Vollmer et al. 2011 ECTRIMS; oral presentation 148). Although the low ARR response has prompted US regulatory agencies to request more efficacy data, several investigators have expressed interest in exploring the value of neuroprotective activity distinct from anti-inflammatory activity for preventing late MS symptoms, such as cognitive loss.
“It is potentially important that both the ALLEGRO and BRAVO studies associated laquinimod with a reduction in the rate of whole brain atrophy when compared to placebo. The slowing of brain atrophy progression in the context of the protection against disability suggests this drug may have neuroprotective properties in addition to its anti-inflammatory effects,” observed Dr. Massimo Filippi, Neuroimaging Research Institute, Vita-Salute San Raffaele University, Milan, Italy.
A study exploring this concept was presented by Dr. Filippi at ECTRIMS. Magnetic resonance images (MRI) taken from MS patients participating in the ALLEGRO trial were analyzed with techniques sensitive to irreversible tissue damage in the white matter (WM) and gray matter (GM). These measures included WM and GM fractions derived from 3D T1-weighted images at 12 and 24 months, magnetization transfer (MT) measures to determine and quantify change in normal appearing WM and GM, changes in proton magnetic resonance spectroscopy and objective changes in Gd+ lesions. All of the MRI parameters evaluated were comparable at baseline between groups.
Several of the findings supported the theory that laquinimod offers at least some degree of neuroprotection. For example, brain volume loss in both WM and GM, which has been correlated previously by other investigators with progression of disability, was reduced in patients treated with laquinimod when compared to those receiving placebo. It also significantly reduced thalamic atrophy at 12 and 24 months relative to placebo, and brain damage was reduced or stabilized on MT analysis.
“Taken together, these findings from a variety of MRI techniques suggest a neuroprotective effect of oral laquinimod in MS patients that is consistent with the delay of disability observed in the ALLEGRO and BRAVO trials,” Dr. Filippi reported. He suggested that the potential for neuroprotection from this agent as well as other agents suspected of acting both through anti-inflammatory and neuroprotective pathways should be pursued not only for its clinical implications but for better understanding MS progression.
Phase III TOWER Data
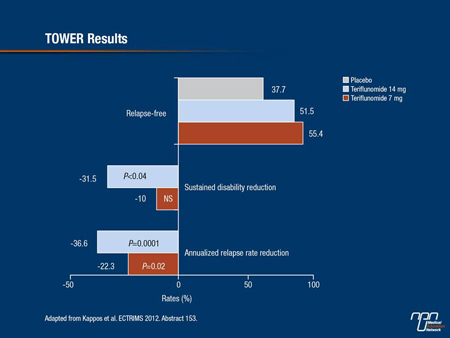
The phase III TOWER data with teriflunomide were presented as a late-breaker at the 2012 ECTRIMS. Although the primary outcomes were widely reported when data were released several months ago, this was the first presentation of the data in a scientific forum. In the double-blind TOWER study, which included participating centres in Canada, 1169 patients were randomized to oral teriflunomide 7 mg once daily, 14 mg once daily or placebo. The primary end point was ARR. The average duration of follow-up was 18 months.
Relative to placebo the ARR was reduced by 22.3% (P=0.02) in the 7 mg group and 36.3% (P<0.0001) in the 14 mg group (Figure 2). The proportion of patients free from relapse at the end of the study was 37.7% in the placebo group, 55.4% in the 7 mg group and 51.5% in the 14 mg group. In addition, the 14 mg dose was associated with 31.5% reduction (P=0.04) in sustained disability at 12 weeks, which was identified by the principal investigator, Dr. Ludwig Kappos, University Hospital, Basel, Switzerland, as a key secondary end point. The reduction in sustained disability was not significant for the 7 mg dose.
Figure 2.
“The activity of the 7 mg dose of teriflunomide was disappointing in this study relative to that seen in previous clinical trials, but the 7 mg and 14 mg doses were similarly well tolerated, which is why the manufacturer is going forward with the higher dose,” Dr. Kappos reported. He noted that in addition to the recent FDA approval granted largely on the basis of this study and a previous phase III study called TEMSO, a favourable regulatory review in Europe is expected imminently.
Distinctive Adverse Events
While the phase III data collected with each of the oral agents suggests a relatively high degree of tolerability and safety, their differences in the rates and types of specific AEs are notable and potentially relevant to how these agents are prescribed. According to data presented at ECTRIMS, the most distinctive AE for teriflunomide includes alopecia, which led to discontinuation in 13.5% of those randomized to the 14 mg dose vs. 4.4% for those randomized to placebo. This AE has not been associated with the other oral therapies that have reached phase III trials.
For BG-12, the most distinctive and common AE has been flushing. In the DEFINE study, this was reported in 38% and 32% of those on the t.i.d. and b.i.d. regimens vs. 5% of those on placebo. However, only 2% and 1% on the t.i.d. and b.i.d. regimens discontinued therapy as a result of this AE, which is relatively mild and is most frequent during the first month of therapy.
For laquinimod, the rate and types of AEs have been similar to placebo, although greater rates of liver enzyme elevations and back pain were reported in both of the phase III trials. The liver enzyme elevations have typically been transient, while there is no clear mechanism for an increased rate of back pain. Discontinuation rates for this or any other AE have been low. The long-term risk posed by laquinimod or any of these therapies for immune-related complications, such as infection or malignancy, awaits longer experience in a larger number of patients, but no substantial concern has been raised by the data collected so far.
Summary
The development of effective, safe and well tolerated oral therapies for MS are widely regarded as clinically important. While the injectable disease-modifying therapies, such as the interferons and GA, have a long efficacy and safety record, oral treatments, which are simpler and easier to administer, are widely preferred by patients. The differences in the mechanisms of action of BG-12, laquinimod and teriflunomide relative to each other and to the first approved oral agent fingolimod may be useful in efforts to better understand the molecular processes that underlie MS while providing a broader array of options for patients who do not adequately respond or tolerate alternatives. The potential for newer agents, including both oral and newer injectable drugs, to provide neuroprotection as well as anti-inflammatory effects may yield an even more important clinical development if neuroprotection reduces the risk of progressive disability.