Reports
Ground-breaking Science in Seasonal Influenza Arms the World to Fight Next Pandemic
This report is based on medical evidence presented at sanctioned medical congress, from peer reviewed literature or opinion provided by a qualified healthcare practitioner. The consumption of the information contained within this report is intended for qualified Canadian healthcare practitioners only.
MEDICAL FRONTIERS - Options X for the Control of Influenza (ISIRV 2019)
Singapore / August 28-September 1, 2019
Singapore – The next influenza pandemic is “a matter of when not if,” according to the World Health Organization (WHO) in its Global Influenza Strategy 2019–2030. Despite seven decades of advances in flu research, substantial barriers remain to preventing this devastating global health threat. Potential solutions cross many boundaries. Options X for the Control of Influenza, the world’s largest flu meeting, provided a unique cross-disciplinary collaboration that leveraged the inter-connectedness of seasonal and pandemic influenza research. Excellent seasonal influenza science, said speaker after speaker, provides a solid foundation for pandemic preparedness. The key focus during the meeting was on vaccines and the urgent need to improve effectiveness, speed up production, increase uptake and broaden options for vulnerable people. Hot topics, covered in over 50 presentations and posters, included an avalanche of data on the performance of non-egg based vaccines and reassuring new clinical data on advanced approaches such as adjuvanted and high-dose vaccines.
Chief Medical Editor: Dr. Léna Coïc, Montréal, Quebec
“For much of the world seasonal influenza vaccine is not seen as a major problem…they only see a pandemic as a problem,” said Dr. Martin Friede of the WHO in his keynote address. “But preparing for a pandemic without having good preparedness for seasonal is almost impossible.” A key part of pandemic prep is the need to improve current flu vaccines, he said, citing the WHO Global Influenza Strategy 2019-30, which calls for vaccines that have better, broader and longer protection and decreased production time. “Why are we still only getting 50 percent [efficacy], why is the duration so short?,” Dr. Friede said.
The false dichotomy between seasonal and pandemic efforts was also a theme for Prof. Kanta Subbarao, Director of the Peter Doherty Institute for Infection and Immunity, Australia. “We shouldn’t forget that a severe epidemic can cause as much of an impact as a pandemic,” she said, citing the 2017-18 flu season, which “caused almost as much influenza-like illness as the 2009 [H1N1] pandemic.”
Dr. Dan Jernigan of the U.S. Centers for Disease Control (CDC) encouraged manufacturers to continue to invest in new vaccine technology. He said that a 5% increase in vaccine effectiveness has the potential to prevent 1 million illnesses and 25,000 hospitalizations in the U.S. (see Figure 1). “So that’s a significant amount of disease that could be prevented with a rather modest increase in the vaccine effectiveness,” he said.
Figure 1.
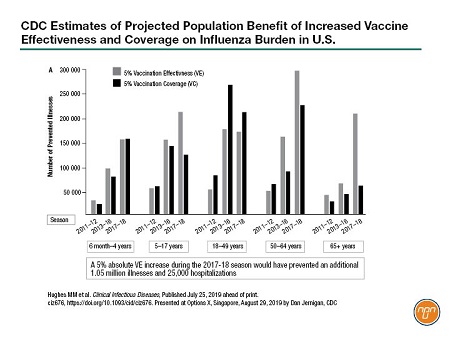
New strides in vaccine technology were a key focus of Options X. Over 40 presentations and posters explored non-egg-based approaches such as cell-based and recombinant vaccines and the use of adjuvants and high-dose formulations to increase vaccine effectiveness in vulnerable populations. Several research teams also presented novel approaches to improve vaccine uptake. This report focuses on these aspects of Options X.
Non-egg-based Vaccines
“We love the egg; the egg has done a fantastic job, but we need to move on and make something that’s safe and effective,” said Dr. Catherine Moore, a consultant clinical scientist for Public Health Wales, U.K., in an interview (see Q&A).
The first cell-based vaccine, approved by the U.S. FDA in 2017, was against H3N2. By 2019, cell-based vaccines against all four strains were available and approved in several countries. Not surprisingly, comparisons of cell-based and egg-based environments were a lively focus during Options X.
One poster was on serological responses to H3N2 A/Hong Kong/4801/2014 (HK 14) strain in 244 Gambian children following immunization. Dr. Ruthiran Kugathasan from Imperial College London and colleagues found that nasal mucosal IgA responses to cell-cultured virus were equal or greater than for egg-adapted HK14. The authors concluded that “further A(H3N2) effectiveness data in young children given live-attenuated influenza vaccines are required, given patterns of serum and mucosal antibody responses observed.”
Broadening the scope, a team from Seqirus, headed by Dr. Sankarasubramanian Rajaram, provided a poster update to a retrospective analysis of publically available data from the Worldwide Influenza Centre, London, for the 2013-18 influenza seasons. The group previously released data showing that circulating H3N2 was more antigenically similar to cell- than to egg-derived reference viruses. The poster at Options X looked at B/Victoria (BVic) and B/Yamagata-lineage (BYam) strains. As expected, since BVic viruses closely resemble H3N2 strains, circulating BVic was more antigenically similar to cell-derived than to egg-derived strains. A less discernable pattern was seen for BYam.
Several posters examined the impact of cell-based vaccines out in the real world. Van Hung Nguyen of VHN Consulting Inc. collaborated with clinicians in the U.K. and Spain to evaluate the cost-effectiveness of cell- versus egg-based quadrivalent influenza vaccines (QIVc and QIVe) during the 2017-18 season. Cell-based vaccines were more cost-effective and reduced disease burden in both countries. In U.K. adults, for example, QIVc “can reduce on average 158,300 symptomatic flu cases, 18,300 outpatient visits, 410 hospitalizations and 66 deaths.” For Canada, Van Hung Nguyen et al. carried out a modelling analysis of vaccine effectiveness using data for the 2011–18 seasons. It predicted that QIVc would be 18%, 26% and 10% more effective than QIVe for children, adults and the over-65s, respectively, in the Canadian setting. Both studies were supported by Seqirus.
Presentations on recombinant vaccines for seasonal influenza largely focused on immune- response updates. For example, US developer Novavax presented results for seven different test formulations of its recombinant saponin-adjuvanted (Matrix-M1) seasonal quadrivalent hemagglutinin nanoparticle vaccine (qNIV). At day 28, three of the Novavax recombinant vaccines induced 17–48% greater HAI titers than Fluzone.
Speed of Manufacture
Speed of manufacture is a point in favour of cell-based vaccines, especially in the setting of a pandemic, said Prof. Subbarao in her keynote address. Egg-based manufacture is “a very logistically challenging task,” she said, and reminded her audience that 95% of currently licensed vaccines are egg-based.
Prof. Steven Riley and team from Imperial College, London, presented a poster reminding delegates that “existing technology and production capacity is expected to produce 400 million doses after a 180-day lead time.” The Imperial College team modelled a moderately severe flu pandemic and calculated that approximately 400,000 deaths could be averted if the production delay was reduced from 180 days to 90 days, and more than 600,000 deaths if production started within 7 days.
During OPTIONS X, several researchers shared updates on accelerating cell-based vaccine production. A poster from Seqirus updated delegates on its large-scale, high-throughput Madine-Darby Canine Kidney (MDCK)-cell-based system and hemagglutinin (HA) yield “leader boards” for each of the key flu viruses. So far, B strains were the highest yielding, followed by H3N2 strains and H1N1 strains (the lowest). Dr. Tsai-Chuang Weng from the National Institute of Infectious Diseases and Vaccinology in Taiwan presented a poster showing increased productivity of MDCK-cell-based vaccines using a suspended cell line. The Taiwan team’s focus is now on scalability of the technology, since peak HA titres of H7N9 fell when the process moved from a 5L to a 50L bioreactor.
Influenza Vaccines for Vulnerable Populations
Older adults bear a disproportionate burden of influenza, said Dr. Melissa Andrew, an associate professor of geriatric medicine at Dalhousie University in Canada, during an OPTIONS X podium presentation. She presented data from Canada’s Serious Outcomes Surveillance (SOS) network. “The real goal of the Network is to measure success of vaccine in older adults,” Dr. Andrew said. During Canada’s 2017-18 flu season, 76% of influenza hospitalizations were in the over-65s; however, vaccine effectiveness declines with frailty, rather than with older age per se, she said. Hospitalization for flu triggers a drastic functional decline in many older adults, from which 5% never recover. Several countries, including Canada and Australia, make high-dose and adjuvanted trivalent influenza vaccines (aTIV) available for older adults. Adjuvanted and high-dose vaccines featured in 30 posters and presentations and were an intense focus of interest at OPTIONS X.
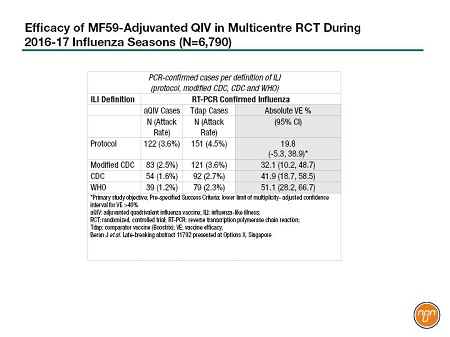
For example, late-breaking results were presented for a large, prospective multicentre efficacy study of an investigational quadrivalent MF59-adjuvanted (aQIV) vaccine, conducted in 12 countries in two consecutive flu seasons – 2016-2017 in the Northern Hemisphere and 2017 in the Southern Hemisphere. The study, involving 6,790 adults over 65, compared the Seqirus aQIV with a non-influenza vaccine comparator (Boostrix) against any PCR-confirmed influenza. Cases were identified based on different definitions of influenza like illness [ILI]. The protocol ILI definition showed a vaccine efficacy of about 20%, a non-significant result. However, as the ILI definition included higher levels of fever in the CDC and WHO definitions, the efficacy increased to 51%, achieving statistical significance (see Table 1). Presenter Dr. Jonathan Edelman pointed out that these efficacy results were achieved despite a 93% mismatched H3N2 season.
Table 1.
Other head-to-head studies included two retrospective analyses of health claims data from the United States comparing Fluzone High-Dose [HD], Fluad [aTIV], and QIV. The first study from Sanofi Pasteur demonstrated a 12% reduction for all respiratory hospitalisation favouring HD over aTIV, generated lively debate on the methodology employed following the presentation. The second set of studies from Seqirus, were conducted using methodologies consistent with recent US CDC studies on influenza vaccine effectiveness in US claims datasets. In these analyses, aTIV was more effective than QIV and HD in reducing influenza related office visits, 36.3% and 16.6%, respectively. With respect to reducing influenza related hospitalisations and ER visits, aTIV was 8.6% more effective compared with QIV, with no difference compared to HD. When evaluating respiratory and cardiovascular related hospitalisations, aTIV was more effective in preventing other respiratory hospital encounters compared to QIV (4.0%) and HD (2.4%). No statistical differences were found between aTIV and HD for reducing other serious cardio-respiratory events or all-cause hospitalizations.
Seqirus fielded a poster with results from a Phase 3, double-blinded RCT comparing its investigational aQIV with its U.S.-licensed aTIV. The study, involving 1,778 people over 65 years old, found the addition of the second B strain in the aQIV did not adversely affect the immunogenicity response against the other strains.
Other investigators provided posters on comparisons between immunogenicity in adjuvanted-and non-adjuvanted vaccines. Athena Pui Yee Li and colleagues at the University of Hong Kong compared standard-dose inactivated (IIV), MF59-adjuvanted, high-dose HA and recombinant-HA vaccines in Hong Kong older adults. The Hong Kong team found that, although HA titres were similar for the four vaccines, the adjuvanted-vaccine antibodies bound to the target with greater avidity and the adjuvanted vaccine induced higher levels of high-avidity antibodies, “so the levels of antibody may not necessarily be different but the adjuvanted vaccine has better quality antibodies that can bind to its target with better strength,” Pui Yee Lee explained. The team also found that T-follicular helper cell levels had a good correlation with antibody responses in the adjuvanted and high-dose vaccines but not in the standard-dose vaccine. “So it seems that the high-dose and adjuvanted vaccines are overcoming this immunosenescence in the elderly,” concluded Pui Yee Lee.
Prof. Robert Booy from Australia’s National Centre for Immunisation Research and Surveillance (NCIRS), reminded delegates during a symposium that children are also vulnerable during flu season. Unlike for other infections, half of all influenza infections in children under five years of age occur in otherwise healthy children and one in eight hospitalizations result in death for this group. During the same GSK-sponsored event, Dr. Halima Tahrat presented results for the Phase 3 trial of a GSK quadrivalent-inactivated flu vaccine (D-QIV) tested in 12,018 children aged 6-35 months, the first results of which came out last year. Vaccine efficacy was 50% for any influenza and 63% for moderate-to-severe influenza.
Seqirus presented updated information on the pivotal pediatric trial of its aQIV versus Fluzone. To assess the effectiveness of revaccination with the aQIV, 1,208 children from the original study were revaccinated the following year. “The conclusion is that, yes, MF59-adjuvanted vaccine can be used for revaccination and it is better than unadjuvanted vaccine,” said author Professor Timo Vesikari of the University of Tampere, Finland. “The results support the use of a quadrivalent adjuvanted vaccine for annual vaccination in young children.”
Questions and Answers
Questions and answers with Dr. Catherine Moore, a consultant clinical scientist for Public Health Wales, U.K., and Dr. Nusrat Homaira, a senior lecturer and respiratory epidemiologist at the University of New South Wales, Australia.
Q: Ongoing genetic drift in both H1N1 and H3N2 in human populations is commonplace. Why the concern around egg-based mutagenesis?
Dr. Catherine Moore: It’s a man-made mismatch. Essentially you’re taking a human virus, which has evolved along with humans and putting it into an avian system to allow it to propagate. What you’re hoping is that it won’t affect the antigenic binding site during that propagation. However, we’re now seeing changes to the antigenic site, especially in H3N2.
Q: What are the clinical implications?
Dr. Catherine Moore: We’re very disheartened when we look at the VE [vaccine effectiveness] data; basically, for H3N2 in the last few seasons it hasn’t worked at all.
Q: How did you feel when you started to see the data on cell-based vaccines?
Dr. Catherine Moore: When you give someone a cell-based vaccine the immune response is much more similar to what’s circulating, so it’s very hopeful, I’m pleased to see it.
Q: Why did you do a study on vaccine uptake in children?
Dr. Nusrat Homaira: Hospitalization for flu in children with chronic lung conditions is four times higher than in children without lung conditions. Yet, vaccine uptake in this group is only 30 to 40%, despite Australia being a developed country where the vaccine is recommended and free for them.
Q: What were the barriers to vaccine uptake in these high-risk children?
Dr. Nusrat Homaira: If children come to the [Sydney Children’s Hospital’s] clinic during the flu season they’re offered the vaccine, but if it’s not during the flu season the staff just recommend it. No systematic reminder is sent to them during the flu season.
Q: What did you do and what were the results of your pilot study?
Dr. Nusrat Homaira: We sent the intervention group [n=23] automated, personalized text-message reminders to encourage uptake. The control group [n=23] did not receive reminders. It was a pilot not powered to show a significant difference, but the difference in vaccine uptake was 61% versus 91% – a 30% absolute increase. We were expecting some increase but this was huge.
Q: How could this change practice?
Dr. Nusrat Homaira: Our conclusion was that this could easily be incorporated into routine clinical care, especially in high-income countries with specialized clinics, but with smartphone technology this could potentially be replicated anywhere. It was very cheap. Sending text messages twice a month for five months cost only AU$800 plus AU$40–50 per child for the vaccine. Compare that to AU$9,000–10,000 for hospitalization.