Reports
New Horizons in the Treatment of Hemophilia A and B
This report is based on medical evidence presented at sanctioned medical congress, from peer reviewed literature or opinion provided by a qualified healthcare practitioner. The consumption of the information contained within this report is intended for qualified Canadian healthcare practitioners only.
MEDICAL FRONTIERS - XXIV Congress of the International Society of Thrombosis and Haemostasis (ISTH)/ 59th Annual SSC Meeting
Amsterdam, The Netherlands / June 29–July 4, 2013
Amsterdam - Current treatment for patients with hemophilia involves intravenous injection of clotting factor concentrate either given on demand in response to a bleeding event or as prophylactic therapy. Since the introduction of recombinant products, the standard of care has become focused on prophylaxis. Despite improvements in the safety of treatment and management of bleeding, currently available products are limited in a number of ways: short half-life; frequent development of inhibitors that inactivate replacement therapy; and the need for repeated venous access. Recombinant DNA technology is being extended to further enhance replacement therapy by producing factors with prolonged half-life and reduced immunogenicity. Targeted bioengineering strategies directed at improving coagulation factors include chemical modification (PEGylation, polysialylation) and fusion protein technologies. A number of new products have already shown safety, prolonged half-life, and delayed clearance in clinical trials, and the most advanced are pending regulatory approval in countries worldwide. The latest data on some of these new hemophilia therapeutics were presented at the congress.
Chief Medical Editor: Dr. Léna Coïc, Montréal, Quebec
Current therapy for hemophilia is based on the infusion of clotting factor concentrates either at the time of bleeding (on-demand therapy) or in a prophylactic schedule to prevent bleeding episodes. The introduction of recombinant products with improved safety has gradually moved the focus of care from management of acute bleeding episodes to prevention of bleeding and management of disease sequelae. Prophylaxis for severe hemophilia is now the standard of care, preferably starting early in life, before the onset of severe hemarthroses, and typically between ages 1 to 3 years. Currently hemophilia A patients administer rFVIII 3-4 times per week and hemophilia B patients use rFIX 2-3 times per week to maintain circulating FVIII or FIX levels above 1% of normal (1 IU/dL), which protects against most spontaneous bleeding episodes. However, despite favorable efficacy, tolerability, and safety profiles, hemostatic protection with current recombinant products is limited in a number of ways: first, development of inhibitors render therapy ineffective occur in approximately 30% of severe hemophilia A patients, less frequently (1-3%) in hemophilia B patients; second, by the short half-life of rFVIII (8-12 h) and of rFIX (18-24 h); third, the necessity for frequent intravenous administration and hence repeated venous access. The requirement for frequent injections puts a burden on patients to maintain a rigorous prophylactic treatment schedule, potentially impacting adherence and quality of life. Adherence to prophylactic regimens is low in many age groups (Berntorp E. Hemophilia 2009;15:1219-27) and substantial morbidity in hemophilia remains (Fogarty PF. Hematology Am Soc Hematol Educ Program 2011:1:397-404).
New long-lasting therapies may have the potential to increase the adoption of prophylaxis, improve adherence to prophylaxis, and improve clinical outcomes. Long-lasting replacement factors may reduce the frequency of infusion such that ports may no longer be required in young children, and a reduction in dosing frequency and flexibility of dosing may improve compliance and adherence to therapy. The more favorable pharmacokinetics of the longer-lasting replacement factors may further reduce the morbidity that results from recurrent hemarthroses and thereby improve the quality of life of patients with hemophilia. Extending the half-life of rFVIII and rFIX has been a major focus of current efforts to improve therapy. One of the leading approaches is fusion protein technology where the clotting factor is linked to another protein with a much longer plasma half-life, such as the fragment crystallizable (Fc) region of IgG or albumin. The genetic fusion of the Fc domain from human immunoglobulin (IgG1) utilizes the endogenous IgG pathway in which the Fc domain binds with neonatal Fc receptor (FcRn), which protects Fc-containing molecules from catabolism, facilitates their recycling thus extending their plasma half-life. Two long-lasting Fc fusion protein products, rFVIIIFc and rFIXFc (Biogen Idec), consisting of a single molecule of rFVIII or rFIX covalently linked to the Fc domain of IgG1, with no intervening sequence is produced in HEK 293 cells, enabling human glycosylation patterns. They are already under regulatory review in several countries for treatment of hemophilia A and B. Albumin is the most abundant protein in the human body and genetic fusion of clotting factors to albumin may extend their half-life. CSL654 (CSL Behring) is a genetic fusion of albumin to the C-terminus of recombinant FIX (rFIX) via a cleavable linker peptide derived from the FIX activation sequence.
Another half-life extension strategy under investigation is through chemical modification of rFVIII or rFIX with polyethylene glycol (PEGylation). The covalent attachment of PEG to available surface groups on the protein, either randomly or through site-specific binding to free cysteine residues or via protein engineering, increases drug efficacy. It is believed this is through increased molecular size, reduced glomerular filtration by masking the protein surface, and decreased immunorecognition response. PEGylation of proteins has been widely reported to be safe and well tolerated. Targeted PEGylated compounds in clinical trials for the treatment of hemophilia A include BAX855 (Baxter) a PEGylated recombinant FVIII (rFVIII) conjugate based on the modification of full-length rFVIII; BAY 94-9027 (Bayer) a B-domain-deleted (BDD)-rFVIII containing a specifically-targeted polyethylene glycol (PEG) molecule; N8-GP (Novo Nordisk) a glycoPEGylated rFVIII consisting of a PEG molecule that has been targeted to an O-linked glycan within the truncated B-domain; N9-GP (Novo Nordisk) a glycoPEGylated rFIX consisting of a PEG molecule that has been conjugated to the FIX activation peptide.
Recombinant Factor VIII Fc Fusion Protein
Hemophilia A, low or absent FVIII anticoagulant activity, occurs in 1 in 5000 males and is the most common hereditary coagulation disorder, affecting 80-85% of hemophilia patients. After 20 years of treatment with rFVIII, new therapeutics are emerging for the treatment of hemophilia A. The results of a Phase III trial with a new, long-lasting rFVIIIFc product were presented by Dr. J. Mahlangu, Director of the Haemophilia Comprehensive Care Center (HCCC), University of Witwatersrand and NHLS, Johannesburg, South Africa at the congress. The open-label, multinational A-LONG trial enrolled 165 previously treated male subjects, median age 30 years, with severe hemophilia A, and no history of FVIII inhibitors. Over a median treatment duration of 30.5 weeks they received either individualized prophylaxis with rFVIIIFc 25-65 IU/kg every 3-5 days, weekly prophylaxis with 65 IU/kg, or episodic treatment with 10-50 IU/kg. PK parameters measured in a sequential subgroup of 28 patients on individualized prophylaxis were comparable with those reported in Phase I/II trial (Powell JS et al. Blood 2012;119:3031-7) and showed consistent improvement vs. rFVIII (Advate). Elimination half-life was 50% longer for rFVIIIFc (19.0 h) compared with rFVIII (12.4 h). Mean time to 1% FVIII activity following rFVIIIFc 50 IU/kg was 4.9 days vs. 3.3 days with rFVIII. The median dose with individualized prophylaxis was 77.9 IU/kg/week at a dosing interval of 3.5 days, and during the last 3 months of the study, 30% of subjects achieved a median dosing interval of 5 days. “The extended PK profile confirmed in this study creates new opportunities for managing patients,” Dr. Mahlangu predicted.
The median annualized bleeding rate (ABR) was significantly reduced for individualized and weekly prophylaxis to 1.6 and 3.6, respectively, vs. 33.6 for episodic treatment (Figure 1); and 45.3% of patients on individualized and 17.4% on weekly prophylaxis experienced no bleeding episodes. These results were not affected by pre-study regimen, bleeds in the 12 months prior to the study, or number of target joints.
Figure 1. Primary End Point: Annualized Bleeding Rates
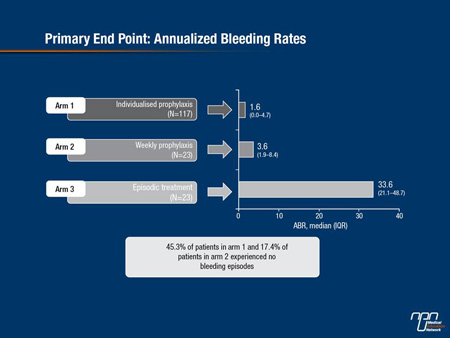
“There is no single standard of care currently to recommend for acute bleeding episodes in patients with hemophilia A,” acknowledged Dr. A. D. Shapiro, Indiana Hemophilia & Thrombosis Center, Indianapolis. Guidelines for initial and follow-up treatment are based on patients’ clinical situations, baseline factor levels, population and individual PK, inhibitor status, and regional economic resources (NHF MASAC document #179, 2007). Studies of currently available treatments report bleed resolution rates of about 71-81% with a single injection. A total of 757 bleeding episodes, mostly spontaneous joint bleeds in patients on episodic treatment, were evaluated in 106 subjects enrolled in the A-LONG study. Most of the bleeds (74.6%) were treated within 8 h from the onset of bleeding. Almost all bleeds (97.7%) were resolved with 1-2 injections of rFVIIIFc; a single injection of rFVIIIFc was sufficient to achieve resolution of a bleed in 87.3%. “This shows improved results over a recent trial of rFVIII in which higher percentages of patients required a higher number of infusions,” Dr. Shapiro noted (Valentino LA et al. J Thromb Haemost 2012;10:359-67). These results were consistent across treatment arms in the study and for patients who were not compliant. The median number of injections required for resolution of a bleeding episode was consistently 1.0 across treatment arms, regardless of type or location of bleed, or time of administration of treatment. The median dose per injection required for resolution of bleeding was 27.4 IU/kg, similar to the median total dose (28.2 IU/kg). Doses required to treat spontaneous and traumatic bleeds were similar. Patient assessment of response to treatment with rFVIIIFc was excellent or good for 78.1% of injections. Physicians’ global assessment of response to the assigned rFVIIIFc regimen was rated as excellent or effective for 99.3% of subjects. “These data give further support to the primary efficacy results from the A-LONG study,” Dr. Shapiro concluded. “The adverse event profile was generally consistent with that expected in patients with hemophilia A,” Dr. Mahlangu noted. rFVIIIFc was well tolerated, the most common adverse events were nasopharyngitis, arthralgia, headache, and upper respiratory tract infection. One treatment-related case of mild rash led to study discontinuation. No cases of anaphylaxis or serious vascular thrombosis were reported. No serious adverse events were assessed as related to rFVIIIFc. No patient developed inhibitors to rFVIIIFc.
An investigational recombinant single-chain FVIII product (rVIII-SingleChain; CSL627) demonstrated a longer half-life than octocog alfa and is well tolerated in patients with severe hemophilia A, according to data presented from the AFFINITY study. Prof Ingrid Pabinger-Fasching, MD, Medical University of Vienna, Austria, explained that rVIII-SingleChain contains a covalent bond linking the light and heavy chains that exist in other rFVIII products. In the first part of the Phase I/III study, 27 men with severe hemophilia A received a single infusion (50 IU/kg) of either octocog alfa or rVIII-SingleChain. Pharmacokinetic measurements over 72 h showed a mean half-life of 14.1 h for rVIII-SingleChain vs. 12.1 h for octocog alfa. Mean clearance was 2.60 mL/h/kg vs. 3.18 mL/h/kg, respectively. Mean AUC-last was 20.2 vs. 17.0 h*IU/mL. rVIII-SingleChain was generally well tolerated. These data suggested that rVIII-SingleChain may address the need for a hemophilia A treatment with a longer half-life, Prof. Pabinger-Fasching commented.
Perioperative Management
In patients with hemophilia A undergoing major surgery, perioperative factor replacement levels of 80-100% are recommended, with continued treatment in the postoperative period to maintain FVIII levels of 20-40%
(Srivastava A et al; Haemophilia 2013;19:e1-47). Dr. Mahlangu presented new findings from a subgroup of 9 patients who underwent major surgery, mainly joint replacement, during the A-LONG trial. “Maintaining appropriate hemostasis during surgery is challenging,” he acknowledged. Dosing for these patients was determined by the investigator and surgeon, taking into consideration the patient’s clinical status, type of planned surgery, and rFVIIIFc PK profile. In this subgroup, hemostasis was effectively maintained during all surgeries, suggesting that perioperative hemostasis achieved after infusion of rFVIIIFc is comparable to that expected for similar surgeries in people without hemophilia. Perioperative hemostasis with rFVIIIFc was rated by investigators/surgeons as “excellent” or “good” for all 9 surgeries. A single injection of rFVIIIFc (median dose 51.4 IU/kg) was sufficient to maintain hemostasis to the end of the surgical period. “In the beginning, patients required a high dose, but that decreased progressively as the patient entered into the postoperative period,” Dr. Mahlangu reported. On the day of surgery, median daily rFVIIIFc consumption was 80.6 IU/kg, decreasing to 53.8 IU/kg for Days 1-3 and 35.2 IU/kg for Days 4-14 following surgery. “These figures are obviously much better than we would normally see in our clinical trials. The number of injections and consumption was much less than observed in other studies,” Dr. Mahlangu commented. Median estimated blood loss was 15.0 mL during surgery and 0.0 mL postoperatively (post-surgical drainage). “It is important to note that intraoperative and postoperative blood loss was comparable to that observed with similar surgeries in nonhemophiliac patients,” Dr. Mahlangu emphasized. No subjects reported a bleeding episode during the postoperative or rehabilitation periods. Overall, 7 adverse events (AEs) were reported in 4 subjects, all assessed as unrelated to rFVIIIFc treatment. “Most importantly, none of the patients on this surgical arm developed any neutralizing antibodies,” Dr. Mahlangu stated. He pointed out that investigators were able to successfully manage these patients using local laboratory monitoring for FVIII activity. “One of the challenges we faced was that rFVIIIFc is a new drug and we wondered whether local laboratories might not be comfortable in doing the measurements. When we correlated the measurements that were done locally, with those done centrally, they were almost all identical. So the lesson here is that you can monitor product locally,” he advised.
Pharmacokinetic Model
A new population PK model developed to aid dosing regimen selection and adjustment in the treatment of hemophilia A patients with rFVIIIFc was applied in the surgical A-LONG patients. Dr. Mahlangu and his colleagues believe that this model is especially useful in formulating general dosing guidance to achieve the high target plasma FVIII activity levels recommended for perioperative management in patients with hemophilia A (Srivastava A et al; Haemophilia 2013;19:e1-47). The 2-compartment model was developed using baseline data from 16 subjects in the Phase I/IIa study and 164 subjects in the Phase III A-LONG study with rFVIIIFc. In the first population PK analysis, the model was found to adequately describe the observed activity-time profile of rFVIIIFc in the 2 studies, with von Willebrand factor (VWF) on clearance identified as the strongest covariate for predicting FVIII activity. When compared with a similar model developed from clinical trial data on rFVIII, the clearance of rFVIII was seen to be 53% higher than the clearance of rFVIIIFc activity, indicating the longer duration of activity with rFVIIIFc. Inter-individual variability was relatively low, underlining the consistency and homogeneity of the activity profiles. A low inter-occasional variability indicated that rFVIIIFc maintained stable and predictable activity with long-term administration over time. When the model was applied to predict rFVIIIFc activity levels during surgery in the A-LONG surgery patients, good correlation was seen between observed FVIII activities during the perioperative period and those predicted by the PK model based on the patients presurgery baseline PK. The model was used to evaluate different dosing scenarios in terms of peak, trough, and average FVIII activity. This model can be validated further and used in the surgical setting or in any other intervention that is required in these patients, according to Dr. Mahlangu.
Long-lasting vs. Short-acting rFVIII
In the absence of comparative clinical trials, mathematical models can be used to compare prophylactic consumption and effects of longer acting and shorter half-life (SA)-FVIII products in hemophilia A. ‘It is very challenging from the payers perspective to calculate what the impact of introducing such products might mean for the future and they often rely on such models’ explained Dr. A. H. Miners, Department of Health Services Research and Policy, London School of Hygiene and Tropical Medicine, UK. Dr. Miners and his colleagues have developed a simple decision-analytic model to compare projected prophylactic FVIII consumption and effects with rFVIIIFc and SA-FVIII products. Applying this model to A-LONG patients suggested that use of an rFVIII with a longer half-life has the potential to reduce the number of injections required and overall factor consumption, without compromising hemostatic efficacy. The model was adapted from 2 PK models of SA-FVIII developed by Collins et al. Cardiff University, UK (Collins PW et al. J Thromb Haemost 2009;7:413-40; 2010;8:269-75), which are linked to the new model using adult half-life data from the A-LONG trial. The model was varied by dose, dosing interval, half-life, and adherence and the results were projected over 1 year for an individual 30 year old receiving SA-FVIII or rFVIIIFc at 37.5 IU/kg infused 3 or 2 times per week, respectively. The results of the analysis suggested similar bleeding frequencies with the 2 products (2 bleeds per year), but 33% less clotting factor was needed with rFVIIIFc compared with SA-FVIII. This effect persisted long-term with a reduction in use, with rFVIIIFc, predicted to be 4.6 million IU over 30 years. Applying A-LONG trial data that more closely reflected dosing schedules from existing clinical trials resulted in a saving of 0.2 million IU when rFVIIIFc was used with a similar frequency of bleeding. “It is a no-brainer that you are going to use less clotting factor, but the main interest is the extent of the difference,” Dr. Miners said. He cautioned that these findings did not come from a clinical trial but a mathematical model. “The amount of clotting factor use that you actually save is very sensitive to the half-life, the time horizon, and the actual dosing schedule used, so there are hundreds of different combinations that you can put into the model. There isn’t one way of doing prophylaxis and this is one of the problems with doing these models,” he said. “However, the model gives reason to believe that with long-lasting rFVIIIs there may be considerably reduced clotting factor use without adverse effects on bleeding levels,” he concluded.
Human-cl rhFVIII
An ongoing clinical trial with human cell line rFVIII (human-cl rhFVIII, Octapharma), a B-domain deleted, human cell-line derived rFVIII concentrate produced in genetically modified human embryonic kidney (HEK) 293F cells, was reported by Dr. Ri Liesner, Director of the Haemophilia Centre, Great Ormond Street Hospital for Children, London, UK. Human-cl rhFVIII is the first rFVIII with human-like post-translational modifications (PTM) and does not contain antigenic residues (e.g., NeuGc or Gal-alpha1,3-Gal) that are present in rFVIII from non-human cell-lines like CHO or BHK derived products. Due to the absence of immunogenic epitopes seen in recombinant FVIII concentrates from hamster cell lines, Human-cl rhFVIII is thought to be potentially less immunogenic. Five prospective studies conducted in 135 adults and children with severe hemophilia A indicated that it is bioequivalent to rFVIII-FS (both analyzed by the one-stage and the chromogenic assay) and effective in the treatment and prophylaxis of bleeding episodes. There were no product-related serious adverse events reported in these studies and none of the previously treated patients developed an inhibitor. In two Phase II/III studies a total of 54 previously treated adult patients with severe hemophilia A and no history of inhibitors, 1016 bleeding episodes were treated, most with one (91.4%) or two (5.8%) infusions. The median dose for treatment of a bleed was 30.9 IU/kg. In 94.4% of patients, efficacy was assessed as “excellent” or “good”. No drug-related “serious” or “severe” adverse events and no allergic reactions were reported. The current international, prospective, open-label clinical trial will assess the product’s immunogenicity in patients with severe hemophilia A without previous exposure to any FVIII concentrate or FVIII containing products. Secondary objectives are the assessment of the efficacy of human-cl rhFVIII during prophylactic treatment, treatment of bleeds, and in surgical prophylaxis. Safety and tolerability will also be assessed as well as pharmacoeconomic aspects of treatment. The study is due to end in 2018. Meantime, human-cl rhVIII is under regulatory review in Europe for the treatment of hemophilia A.
Recombinant Factor IX Fc Fusion Protein
Hemophilia B, the deficiency or complete absence of FIX, affects an estimated 80,000 people throughout the world, representing about 20% of total hemophilia cases. Because FIX prophylaxis remains underutilized, patients with hemophilia B may be at risk for bleeding that can be severe and potentially life- or limb-threatening, and they may experience arthropathy resulting from recurrent hemarthroses. Patients who start on prophylaxis after the age of 3 years may continue to experience bleeding episodes, although reduced in frequency. Epidemiological data show that some patients still develop joint damage.
Fc fusion technology has also been applied to rFIX to prolong the half-life of treatment in hemophilia B. Clinical trials completed with rFIXFc include a Phase I/IIa study (Shapiro MD et al. Blood 2012;119:666-72) and the Phase III B-LONG study, the results of which were presented by Dr. Jerry S. Powell, Hemophilia and Thrombosis Center, University of California (UC), Davis Comprehensive Cancer Center. “The international study recruited 123 previously treated patients (median age 30 years) with severe hemophilia B and no history of FIX inhibitors. Patients were enrolled onto 1 of 4 treatment arms: weekly prophylaxis (starting at 50 IU/kg every 7 days, adjusted to maintain factor levels); individualized interval prophylaxis (100 IU/kg starting at every 10 days; interval adjusted to maintain factor levels); episodic (on-demand) treatment (20-100 IU/kg); or perioperative management. A 96-hour PK sampling schedule in 22 patients on weekly prophylaxis vs. rFIX, showed a mean half-life of 82.1 h for rFIXFc vs. 33.8 h for rFIX. Analysis using data up to 48 hours yielded median half-life (95% CI) of 17.04 (15.89, 18.26) hours for BeneFIX®, which is consistent with the mean half-life of 18.1 ± 5.1 hours stated in the product monograph. The PK of rFIXFc was stable over repeated dosing. Mean time to 1% FIX trough after a 50 IU/kg dose was 11.2 days for rFIXFc vs. 5.1 days for rFIX. Clearance was improved at 3.19 vs. 6.34 mL/h/kg, respectively. “Importantly, the time to 1% FIX activity was significantly increased with a decrease in clearance for rFIXFc vs. rFIX, out to a median of 11.2 days vs. 6.1 days,” Dr. Powell pointed out. All these differences were statistically significant (P<0.001). “With the extended half-life product we can now conveniently maintain FIX levels higher than 1% [1 IU/dL] for our patients - that is an exciting outcome of the evidence from this clinical trial,” he said. The dosing interval for subjects on individualized interval prophylaxis increased from the initial 10 days to ≥14 days in 53.8% of subjects during the last 3 months of the study.
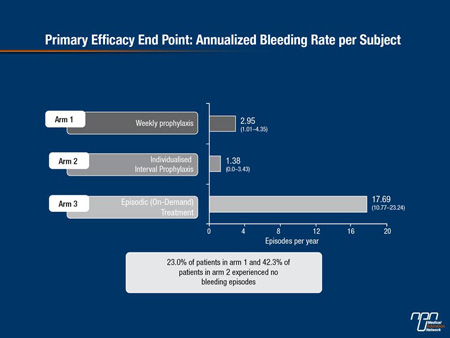
Markedly reduced ABRs were seen in the patients on weekly and individualized prophylaxis compared with those on episodic treatment (2.95 and 1.38, respectively, vs. 17.69) (Figure 2). Among the patients on weekly (fixed dose) and individualized prophylaxis 23.0% and 42.3%, respectively, experienced no bleeding episodes over the entire year of study. “Unsurprisingly, every prespecified subgroup looked at benefiting from the rFIXFc and its improved pharmacokinetic parameters,” Dr. Powell noted. As expected the on-demand group had a high ABR. “Both the group that was on prophylaxis and the group that received on-demand treatment prior to going on study benefited substantially from rFIXFc. Those who had very few bleeds over the year prior to going on study and those who had significant number of bleeds prior to going on study also benefited.”
Figure 2. Primary Efficacy End Point: Annualized Bleeding Rate per Subject
rFIXFc was demonstrated to be safe in B-LONG. The AE profile was consistent with that expected from a typical hemophilia B population.
A total of 636 bleeding episodes were treated in 89 subjects in the B-LONG study, 90.4% of all bleeds were controlled with one injection and 97.3% of all bleeds were controlled by ≤2 injections of rFIXFc. “This control of bleeding episodes was at least comparable to currently available therapies,” said Prof. John Pasi, The Barts and The London School of Medicine and Dentistry, Queen Mary, University of London, UK. The median dose per injection was 46.1 IU/kg and total dose per bleeding episode was 47.0 IU/kg. Overall, subject assessment of response to injections with rFIXFc was “excellent” or “good” for 82%, whereas physicians’ global assessment of subject response to their rFIXFc regimen was rated as “excellent” or “effective” for 98.8%. Efficacy was consistent across all subgroups and no subjects had responses rated as ineffective at any visit. “These findings add to the primary efficacy results from the B-LONG study in establishing rFIXFc as an efficacious therapy for both prophylaxis and control of bleeding episodes,” he said.
The clinical efficacy of a once-weekly dosing regimen of recombinant fusion protein linking FIX with albumin (rIX-FP; CSL654) was reported from a Phase I/II study by Prof. Uri Martinowitz, Chaim Sheba Medical Center, Tel Hashomer, Israel. rIX-FP is being developed for prophylaxis and treatment of bleeding episodes in hemophilia B patients through a clinical trial program, PROLONG-9FP. In this trial, 17 patients received rIX-FP, 13 as weekly prophylaxis for 11 months, and 4 as on-demand treatment. All prophylaxis patients maintained weekly treatment throughout the study with a mean ABR of 1.26. All bleeding episodes were treated successfully with ≤2 infusions, with 95.3% of events treated with a single infusion. Following a single 25 IU/kg infusion of rIX-FP, Mean FIX activity was 3.8% and 2.7% at day 7 and 14, respectively and half-life was 94 h. People with hemophilia B require FIX infusions 2-3 times per week to achieve a significant reduction in bleeding, Prof. Martinowitz noted. “This trial showed that less frequent infusions were needed with rIX-FP compared to currently available FIX products used on-demand or prophylactically to prevent or treat bleeding episodes,” he said.
Perioperative Management
Dr. Powell also reported on the efficacy of rFIXFc for hemostatic control in 12 B-LONG patients who underwent 14 major surgeries (mainly joint replacements). In these patients, rFIXFc maintained perioperative hemostasis rated by investigators/surgeons as “excellent” or “good” in all cases. “Hemostasis achieved after infusion of rFIXFc appeared to be comparable to that for similar surgeries in subjects without hemophilia,” said Dr. Powell. Median blood loss was as expected with any major surgery, at 65.5 mL during surgery and 0.0 mL postoperatively. No blood transfusions were required during surgery, but two subjects received transfusions in the postoperative period. A single injection of rFIXFc was required in 85.7% of surgeries to maintain hemostasis during surgery, at a median dose of 90.9 IU/kg per injection. Most procedures required 1 to 2 injections the day preceding and the day of surgery, and most subjects required 2 to 3 injections 1-3 days after surgery. For most patients, the total daily dose of rFIXFc decreased on Days 4-14 post surgery. Overall, one or more AE(s) were reported for 10 (83.3%) of the 12 subjects, and 3 subjects reported 6 serious AEs, all of which resolved and were judged as unrelated to rFIXFc treatment. “These are what would be expected in major surgery in patients with hemophilia,” Dr. Powell said. No study patients developed inhibitors, anaphylaxis, or thrombotic reactions. “This is a clear example of how rFIXFc is going to be used – it represents a tremendous saving,” Dr. Powell said. He added that investigators were able to successfully manage patients having major surgery using local laboratory monitoring for FIX activity.
Pharmacokinetic Model
A 3-compartmental population PK model has been developed by US researchers to evaluate dosing regimens of rFIXFc in routine prophylaxis, episodic treatment, and perioperative management of people with hemophilia B (Diao L et al. Hemophilia 2013;19(Suppl 2):33. Abstract PO040). The model was based on PK profiles of 12 subjects in the Phase I/IIa study with rFIXFc (Shapiro M et al. MD et al. Blood 2012;119:666-72) and baseline PK profiles in 123 subjects, and repeat PK profiles in 21 subjects in the B-LONG study. Using this model to simulate regimens for prophylaxis in adult patients predicted that rFIXFc 50 IU/kg once weekly or 100 IU/kg every 10-14 days would maintain FIX activity at ≥1% in the majority of the population. Simulations for control of bleeding episodes showed that a single dose of 50 or 100 IU/kg of rFIXFc was sufficient to achieve FIX peak activity levels of 40-80 IU/dL, and is recommended by the World Federation of Hemophilia (Srivastava A et al. Haemophilia 2013;19:e1-47). Overall, the variability of rFIXFc PK between subjects was small, with body weight being the only covariate that showed a weak association with rFIXFc PK. “Considering the wide therapeutic range for factor replacement therapy, body weight-independent (i.e., flat) dosing of rFIXFc might be a viable approach in adult hemophilia B patients that warrants further investigation,” said Dr. Shuanglian Li, Biogen Idec. Dr. Powell described how the population PK model was applied to simulate FIX activities in the 12 major surgery patients in the B-LONG study and compared with observed FIX activities as measured by central and local laboratories. The FIX activities measured during the perioperative period were largely consistent with the predictions based on subjects’ pre-surgery PK characteristics, indicating no substantial factor consumption during these surgeries. There was good correlation between observed and predicted FIX activity and Dr. Powell and his colleagues believe that this model could be a useful tool in guiding dosing to achieve high plasma FIX activity levels recommended for perioperative management in patients with hemophilia B. “rFIXFc is safe and predictable; if you are experienced with rFIX, you can dose rFIXFc,” Dr. Powell commented.
GlycoPEGylation
The glycoPEGylated rFIX N9-GP (nonacog beta pegol) has been shown to have a significantly longer plasma half-life (93 h) compared with currently available rFIX products (Négrier C et al. Blood 2011;118:2695-701). In Phase I, N9-GP also showed a 10-fold slower clearance, and incremental recovery was almost 2 times higher than for rFIX. The results of the first Phase III trial with N9-GP, an international, multicenter, blinded study of safety and efficacy, were presented by Dr. Peter Collins, Arthur Bloom Haemophilia Centre, Cardiff University School of Medicine, Wales. Two different prophylaxis dose levels of N9-GP (10 and 40 U/kg) were tested, along with an on-demand treatment arm. The results showed that N9-GP was able to regulate the frequency of bleeding episodes safely and without development of inhibitors, although the prophylactic efficacy of the lower dose could not be confirmed. The trial enrolled 74 previously treated patients (mean age 31.4 years) with ≤2% FIX activity, and no history of inhibitors. They were treated with N9-GP on-demand (28 weeks) or blindly randomized to a prophylactic regimen of 10 U/kg or 40 U/kg N9-GP once weekly for 12 months. A bleeding episode was treated with a single dose of 40 U/kg (80 U/kg for severe bleeds) on all trial arms. Following completion of the dosing period, all patients were offered continued treatment with N9-GP in the extension trial. No inhibitory antibodies to FIX were detected by any method. However, low-titer, transient binding (non-inhibitory) antibodies were detected by ELISA in 3 patients, 2 of whom had them at baseline and maintained them throughout the study without any impact on the PK properties or the clinical efficacy of the molecule. Among patients who randomly received 40 U/kg N9-GP, 99% of bleeding episodes were treated with only one infusion, while two-thirds of the patients experienced complete resolution of bleeding in their target joints. Of a total of 60 AEs reported, 12 were considered treatment-related. There were no apparent differences between the treatment groups with respect to AEs and standard safety parameters. No thrombosis or allergic reactions were seen. The PK results confirmed Phase I data with a mean half-life of N9-GP of 107.0 h and 110.8 h for 10 and 40 U/kg, respectively in steady state and FIX trough levels of 0.08 U/mL (10 U/kg) and 0.31 (40 U/kg). Efficacy end points included treatment of bleeds, in the prophylaxis arm 86.9% at 10 U/kg and 97.1% at 40 U/kg was successful compared to 95.1% success for on-demand treatment. For prophylaxis, ABR met the prespecified end point for 40 U/kg (2.5) but did not for 10 U/kg (4.6). Median ABR for patients treated on-demand was 15.6, whereas patients on prophylaxis was 1.0 and 2.9 with 40 U/kg and 10 U/kg, respectively. This was a good result, Dr. Collins noted, as 98% of patients were compliant with prophylactic treatment. Among patients with ≥1 target joint at baseline (10 out of the 15 on 40 U/kg prophylaxis, 1 out of the 13 on 10 U/kg and 2 out of the 12 in the on-demand group had no bleeds during treatment.
Clinical Monitoring
For newly emerging treatments of hemophilia there is a need to identify reliable activated partial thromboplastin time (aPTT) assay reagents and coagulometers that can be used for clinical monitoring of factor activity. A study that compared 5 widely used commercial aPTT reagents for evaluating BAY 94-9027 activity was reported. BAY 94-9027 is an investigational B-domain-deleted rFVIII produced using the same cell line as rFVIII-FS, covalently linked to a single PEG that in preclinical models showed extended efficacy due to prolonged half-life compared with un-PEGylated FVIII (Mei B et al. Blood 2010 116:270-9). In Phase I it showed significantly (1.5-fold) longer half-life and higher area under the curve per dose (AUC/D) than rFVIII-FS (Coyle T et al. Haemophilia 2012;18[Suppl 3]:22. FP-MO-03.2-3). No change in half-life was observed after multiple dosing. BAY 94-9027 is in Phase II/III trials in adults and in children. Early observations have shown 5 bleeding events effectively treated with a single infusion. No inhibitory or non-inhibitory antibodies were detected against FVIII or any other product components. US researchers from Bayer HealthCare reported that using plasma samples from people with severe hemophilia A and 2 different coagulometers, ellagic acid-based aPTT reagents revealed high correlation between assays and instrument, and were therefore deemed the most suitable for evaluating potency of BAY 94-9027 and for use in clinical laboratories for monitoring FVIII:C after infusion. Prolonged clot time and poor precision were seen in silica-based aPTT assays, which may have been due to interference with contact activation on the silica surface by the PEG moiety, the researchers suggested.
PEGylation of proteins could influence the clotting times in certain aPTT based assays, so researchers from Diagnostica Stago have investigated the impact of PEGylation in two factors, N8-GP and N9-GP (Novo Nordisk), on FVIII and FIX activity, respectively, using the one stage clotting assay with a wide range of aPTT reagents on the STA-R® analyser (mechanical clot detection, Diagnostica Stago) and chromogenic assays. N8-GP and N9-GP are 40 kD site-specific PEGylated recombinant human FVIII and FIX derivatives in late stage clinical development for hemophilia A and B, respectively (Tiede A et al. J Thromb Haemost 2013;11:670-8; Négrier C et al. Blood 2011;118:2695-701). Using non-PEGylated molecules (WHO IS or rFVIII) as calibrators, levels of FVIII were underestimated with silica-based APTT reagents and in the normal range with a kaolin- and ellagic acid -based aPTT reagents, and with a FVIII chromogenic assay. Using the N8-GP molecule as calibrator, FVIII levels were all in the normal range, regardless of the aPTT reagent. Using WHO IS or rFIX as calibrators, levels of FIX were underestimated with a kaolin-based aPTT reagent, overestimated with silica-based aPTT reagents, slightly underestimated with an ellagic acid based aPTT reagent, and in the normal range with a FIX chromogenic assay. Using the N9-GP molecule as calibrator, FIX levels were all in the normal range, whatever aPTT reagent used. The study confirmed the impact of PEGylation of FIX and FVIII on FIX and FVIII activity aPTT based assays, respectively, concluded Dr. Anne Lochu. Some reagents provided activity in the normal range on chromogenic assay, while others showed a wide variability. Dr. Lochu suggested that further investigations are needed to understand the biochemical mechanisms involved in these interactions and to try to normalize the results by different means, such as the use of a specific calibrator or assigning an assay specific conversion factor to obtain results comparable to those obtained with chromogenic assays.
Baxter BioScience researchers reported development of a modification-dependent activity assay (MDAA) to selectively measure PEGylated human rFVIII in human plasma. The MDAA combines the use of an antiPEG antibody with a chromogenic factor Xa-based FVIII activity assay. It was successfully validated for BAX855 in human plasma. BAX855 is a PEGylated rFVIII product developed to extend the half-life of rFVIII plasma/albumin-free method (Turecek PL et al. Hämostaseologie 2012;32(Suppl 1):S29-38). The assay’s specificity allowed measurement of BAX855 activity in normal human and FVIII-deficient plasma without interference by endogenous FVIII, with a mean total error of 17.7% at the assay’s LLOQ of 0.07 U/mL and <15% at other BAX855 concentrations investigated. This particular concept of specifically capturing PEGylated rFVIII before measuring its activity can be extended to other PEGylated proteins, the researchers suggested. The results obtained with this newly developed assay type will allow a determination of whether or not the PEGylated protein is still intact.
Monitoring of FIX levels in patients is usually carried out in clinical hemostasis laboratories by the one-stage clotting assay using an aPTT reagent and WHO-traceable pooled normal plasma calibrators. Since significant variability exists between laboratories and the aPTT agents they use, a field study was conducted to compare accuracy and inter-lab variability in measuring rFIXFc vs. rFIX activity in spiked plasma samples at 30 clinical hemostasis laboratories. Blind testing of samples using routine one-stage clotting assay and in-house FIX plasma standard showed considerable overestimation of label activity for mean spike recovery for rFIX, ranging from 121% at 0.8 IU/mL to 167% at 0.05 IU/mL. Median spike recovery for rFIXFc ranged from 88% to 132%. Among the aPTT reagents used in clotting assays, ellagic acid generally resulted in the highest observed activities, showing good correlation between rFIXFc and rFIX. In comparison, silica and kaolin typically resulted in lower observed recoveries for rFIXFc. High inter-laboratory variability was recorded, with CVs ranging from 13% to 31% for rFIX and from 27% to 45% for rFIXFc. The differential reagent-dependent effects on rFIXFc and rFIX observed in the field study were reproducible in a side-by-side comparison at a central laboratory. For both products, the assay results were generally consistent when compared between laboratories using the same reagents and calibrators, with silica-based assays showing on average 80% spike recovery and kaolin 60% recovery for rFIXFc (the worst result), while rFIX recovery at the 0.8 IU/Ml level ranged from 80% to 125% of expected. These findings indicate that further progress towards method and reagent uniformity is needed to improve the accuracy for current rFIX products and to more precisely define and predict the potential impact of assay differences for modified rFIX products.
Real-world Barriers to Treatment
A study that looked at “real-life” experiences with hemophilia treatment were presented by Dr. E. P. Armstrong, Strategic Therapeutics, LLC & College of Pharmacy, University of Arizona, Tucson, Arizona. The study utilized electronic medical records and administration encounters/claims data collected for military service personnel and their families by the US Department of Defense (DOD). “The big advantage of this database was that it contained years of data for the same patients, which is unusual for the United States, where patients tend to move between different managed care organizations,” Dr. Armstrong explained. “We were interested in looking at how patients were actually using hemophilia products,” he said, noting that the benefits of prophylaxis can be compromised by nonadherence to prescribed regimens (Thornburg CD. J Coagul Disord 2010;2:9-14). The retrospective observational study, using data from 2006 through 2011, identified 207 patients diagnosed with hemophilia (74.9% and 25.1% hemophilia A and B, respectively) who received clotting factors at least twice during the study period. The median age of these patients was 7.0 years, since most were children with at least one parent in the DOD. There were 101 (48.8%) mild, 32 (15.5%) moderate and 74 (35.7%) severe hemophilia patients. Medication adherence was assessed using prescription claims for clotting factors by examining up to 10 sequential time periods of 180 days for each patient’s continuous enrollment. Adherence within the time period was calculated using days’ supply for all claims divided by 180 days. Patients were considered adherent within the time period if the ratio of days’ supply to observed days was ≥60%. Patients with severe hemophilia had significantly more time periods when they were at least 69% adherent, based on their days’ supply of clotting factor to observed days, compared with patients with mild or moderate hemophilia (51% vs. 14% and 21%, respectively; P<0.001). “Many of the patients with mild or moderate hemophilia were on on-demand therapy so we would expect adherence to be low, so that was no surprise,” Dr. Armstrong said. Among those with severe disease, however, there was a wide range in adherence, with 66.2% being adherent less than 70% of the time (Figure 3).
Figure 3. Adherence to Clotting Factors Among Persons with Type A or B Haemophilia
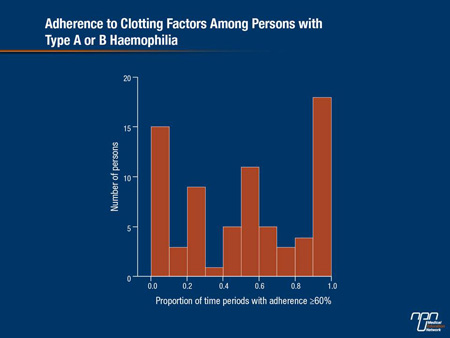
“This surprised us, since a number of previously published registry studies showed higher numbers than this,” Dr. Armstrong admitted. “In real-life practice, we found that among people with severe disease you would think would be on prophylactic regimens, many were not adherent with that regimen.” He added, “People who were most adherent were taking a lot of product, as we expected. What surprised us is that we saw a large number of people with hemophilia who were not getting very much product; that was an important consistent finding.” Dr. Armstrong stressed that this low adherence was not driven by financial barriers, since all drugs were free to these patients. Another unfulfilled expectation was that children would have higher adherence rates due to the care of their parents, he added. “We anticipated that many people would routinely be on prophylaxis and receive therapy routinely and our results showed that this is not the case. This demonstration of very low adherence rates could be clinically important,” he said, “since adherence can be very important to prevent joint damage.” Dr. Armstrong suggested that working with caregivers and the use of different products that are easier to administer may have some benefit.
Summary
For 20 years, the mainstay of hemophilia treatment has been recombinant coagulation factors, which increased the worldwide capacity for replacement therapy and facilitated aggressive prophylactic therapy. Nonetheless, barriers remained to broad application of prophylaxis. These are being addressed by a promising pipeline of novel factors for treatment of hemophilia A and hemophilia B and, as reported at the ISTH and other recent hemophilia congresses, longer-acting versions of rFVIII and rFIX that require less frequent dosing are likely to become available soon in a number of countries. It is hoped that they will reduce the burden of treatment for patients and as a result, adoption of prophylaxis will increase, and adherence to prophylaxis and hence, clinical outcomes will improve. Meantime, the potential for a real cure for hemophilia probably lies in further research into gene therapy.