Reports
New Treatment Options in Pulmonary Arterial Hypertension Appear to Move Treatment Goals Beyond Symptom Control
This report is based on medical evidence presented at sanctioned medical congress, from peer reviewed literature or opinion provided by a qualified healthcare practitioner. The consumption of the information contained within this report is intended for qualified Canadian healthcare practitioners only.
MEDICAL FRONTIERS - European Respiratory Society (ERS) Annual Congress 2013
Barcelona, Spain / September 7-11, 2013
Barcelona - New data presented at the ERS 2013 from trials published just weeks before the meeting was convened, document a paradigm shift in the treatment of pulmonary hypertension. In pulmonary arterial hypertension, the benefit of a new therapy was documented on events rather than on exercise capacity. It is the first time a favorable effect has been demonstrated on morbidity and mortality on PAH in a Phase III trial. The data support a shift in therapeutic goals. Separately, a trial associated a different agent with clinical benefit in inoperable chronic thromboembolic pulmonary hypertension (CTEPH). This is the first Phase III demonstration of a clinically meaningful improvement for any outcome in CTEPH with a pharmacological therapy. Separately and together, the studies document important progress in a set of progressive and incurable disorders.
In patients with pulmonary hypertension (PH), control of pulmonary vascular resistance (PVR) reduces symptoms by improving exercise capacity. However, the value of this mechanism for reducing risk of the morbidity and mortality from PAH was only recently demonstrated in a Phase III trial. New data presented at the ERS from that trial, called SERAPHIN and conducted with the novel endothelin-receptor antagonist (ERA) macitentan (Pulido et al. N Engl J Med 2013;369:809-18), support the value of hard end points in assessing new treatments for PAH.
SERAPHIN: Hard End Point Trial
“The SERAPHIN study has set a new standard in PAH trials as a consequence of its long-term design and unique primary end point, which allows the true progression of PAH to be assessed,” reported Hossein-Ardeschir Ghofrani, MD, University Hospital, Giessen, Germany. Based on this study and another recently published Phase III trial called CHEST-1, which evaluated a first-in-class agent called riociguat for the treatment of inoperable CTEPH, Dr. Ghofrani predicted an evolution in the order of treatments for PH once these new agents receive regulatory approval.
In SERAPHIN, which was updated at the ERS (Ghofrani et al. ERS 2013, Abstract 1786), 742 patients with PAH were randomized to 3 mg macitentan, 10 mg macitentan, or placebo given once daily. After a mean treatment duration of almost two years, the 10 mg
dose of macitentan was associated with a 45% (HR 0.55; 97.5% CI : 0.39-0.76; P<0.001) risk reduction relative to placebo in the primary composite end point of death, atrial septostomy, lung transplantation, initiation of treatment with intravenous or subcutaneous prostanoids, or worsening of PAH.
A significant 30% reduction in the same composite end point relative to placebo was also achieved with the 3-mg dose (HR 0.70; 97.5% CI 0.52-0.96; P=0.01), but the 10 mg dose was equally well tolerated, suggesting it has greater clinical utility. The only potential disadvantage for the higher dose relative to the lower dose was an increased risk of anemia, defined as Hg
≤ 8 g/dL, but this occurred uncommonly in both groups (4.3% vs.1.7%). Overall, macitentan was well tolerated. When compared to placebo, the largest differences in adverse events with either dose of macitentan were for headache (~14% vs. 9%) and nasopharyngitis (~15% vs. 10%).
In addition to the primary end point, macitentan reduced a combined secondary end point of hospitalizations for PAH and death due to PAH by 50% (P<0.001) with the 10 mg dose and 33% (P=0.0146) with the 3 mg dose (see Figure 1). According to Dr. Ghofrani, the relative advantage of macitentan was consistent irrespective of background PAH therapy, which, in most cases involved phosphodiesterase-5 (PDE-5) inhibitors.
Figure 1.
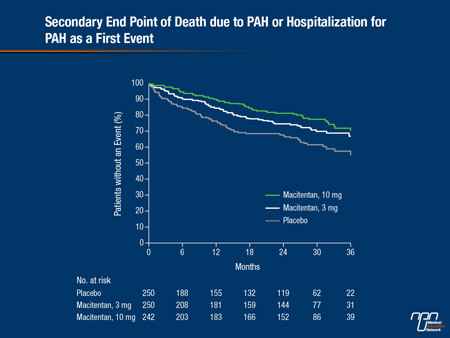
All of the most potent therapies in PAH, which includes PDE-5 inhibitors, prostacyclin and its analogues, and ERAs, reduce symptoms by reducing pulmonary vascular resistance. Prior to the SERAPHIN trial, clinical benefit was established by demonstrating an improvement in the 6-minute walk distance (6MWD) test relative to placebo. Based on evidence that the 6MWD test is not a sensitive predictor of outcome, a consensus group published a recommendation to use hard morbidity and mortality end points in future efficacy trials (McLaughlin et al. J Am Coll Cardiol 2009;54(suppl):S97-107). SERAPHIN is the first study to do so.
“SERAPHIN demonstrates that large-scale studies that evaluate the effect of therapy on morbidity and mortality in PAH are feasible, and this, I think, shifts our attention toward improving long-term outcomes not just improving symptoms,” Dr. Ghofrani reported.
It is unclear whether existing therapies in PAH would provide the same degree of benefit. When compared to other ERA agents, macitentan has demonstrated greater and more sustained receptor binding and greater tissue penetration. In experimental models, for example, the receptor occupancy time of macitentan was 15-fold greater than bosentan, providing reductions in vascular resistance even in the presence of very high concentrations of endothelin (Garfield et al. PLoS One 2012;7:e47662). The dual inhibition of the endothelin ETA and ETB receptors may be relevant to safety.
Dual Receptor Inhibition Considered
“Clinical studies suggest that the dual inhibition of macitentan may reduce the risk that this drug, relative to other ERA agents, will produce edema, which appears to be caused by activation of the plasma vasopressin system via secondary stimulation of the uninhibited ETB receptor,” according to Dr. Sean Gaine, Mater Misericordiae University Hospital, Dublin, Ireland. Dr. Gaine presented safety data on macitentan at the ERS (Gaine, ERS 2013, Abstract 1700).
SERAPHIN hemodynamic studies presented at the ERS further support the potency of this agent in attenuating the pathophysiology of PAH (Sitbon et al. ERS 2013, Abstract 4060). When compared to baseline, placebo-corrected treatment effects for macitentan were statistically significant at 6 months for mean pulmonary arterial pressure, cardiac index, and PVR. The improvements with macitentan relative to placebo were significant for the 10 mg dose independent of functional class or use of another PAH therapy at the start of the trial (see Figure 2).
Independent of the agent studied, SERAPHIN is considered a landmark in the effort to change the natural history of PAH, which is progressive and incurable. While macitentan 10 mg relative to placebo did significantly improve exercise capacity as measured with the 6MWD test at 6 months (P=0.008) and reduce symptoms as measured with WHO functional class (P=0.006), the reduction in key clinical events confirms that the progression of PAH can be attenuated. On macitentan 10 mg, the reduction in death due to PAH fell just short of clinical significant (P=0.07).
Figure 2.
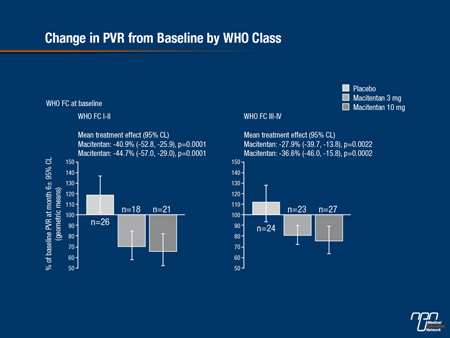
The results of SERAPHIN redefine treatment goals in PAH, but another study also published just prior to the ERS appears to outline an important clinical advance in CTEPH. In the CHEST-1 study, riociguat, a novel first-in-class stimulator of soluble guanylate cyclase (sGC), was found to improve exercise capacity in CTEPH, a benefit never previously demonstrated in a Phase III trial with a pharmacological therapy (Ghofrani et al. N Engl J Med 2013;369:319-29).
“The improvement in exercise capacity at 16 weeks was supported by significant reductions in pulmonary vascular resistance,” reported Dr. Eckhard Mayer, Kerckhoff Heart and Lung Center, Bad Nauheim, Germany. Presenting an analysis at ERS of the relative effect of riociguat in inoperable relative to postoperative CTEPH patients treated in CHEST-1 (Mayer et al. ERS 2013, Abstract 1781), Dr. Mayer reported that significant benefit was achieved in both.
In CHEST-1, unlike SERAPHIN, the primary end point was exercise capacity as measured with the 6MWD test at 16 weeks. There were 261 patients randomized of which 189 had inoperable thrombi and 72 had persistent or recurrent PH after endarterectomy. At 16 weeks, 6MWD was increased by 39 meters in the group randomized to riociguat but was fell by 6 meters in the group randomized to placebo (P<0.001). Although the difference relative to placebo was greater in the inoperable group (54 vs. 26 meters), Dr. Mayer reported that the favorable change was accompanied by improved hemodynamics in both groups.
In separate data presented from an open-label continuation study called CHEST-2, benefit was sustained at one year, and the side effect profile remained favorable (Simmoneau et al. ERS 2013, Abstract 1785). In CHEST-1, the side effects observed more commonly on the active treatment than placebo included headache (25% vs. 14%), dizziness (23% vs. 12%), dyspepsia (18% vs. 8%), nasopharyngitis (15% vs. 9%), and gastrointestinal complaints, such as vomiting (10% vs. 3%), and constipation (6% vs. 1%).
Third Phase III Trial Also Positive
New data from a separate trial with riociguat, called PATENT-1, were also presented at the ERS. That trial, like SERAPHIN and CHEST-1, was also published just prior to the ERS (Ghofrani et al. N Engl J Med 2013;369:330-40), but the significance of these data for advancing care in PH was less immediately apparent relative to those generated by SERAPHIN or CHEST-1. In PATENT-1, patients with PAH were randomized to riociguat or placebo, but 6MWD, rather than clinical events, was the primary end point. Although 6MWD was significantly improved on riociguat relative to placebo (P<0.001), this end point does not necessarily predict long-term benefit. It is notable that new PATENT-1 data presented at the ERS found a weak correlation between change in 6MWD and change in hemodynamics (Galiè et al. ERS 2013, Abstract 1784).
“The correlation between 6MWD and hemodynamics was significant but small. It suggests that assessment of several parameters is necessary to evaluate the overall treatment effect of PAH therapies in the individual PAH patient,” reported Dr. Nazzareno Galiè, Department of Experimental, Diagnostic, and Specialty Medicine, Bologna University, Italy.
While an open-label extension study of patients enrolled in PATENT-1, called PATENT-2, demonstrated further improvements in 6MWD in a median follow-up of 441 days, the SERAPHIN trial establishes a more rigorous event-driven assessment of new agents going forward. This does not diminish the importance of showing improvements in exercise capacity or, perhaps more importantly, health-related quality of life (HRQoL), but it does turn attention to improving long-term outcomes.
In SERAPHIN, secondary outcomes did include exercise capacity and change in functional class. HRQoL, which has the potential to reflect treatment efficacy as well as freedom from adverse events, was evaluated at 6 months in SERAPHIN with the Short-Form 36 (SF-36). These data were originally presented at the American Thoracic Society (ATS) but updated at the ERS (Jansa et al. ERS 2013, Abstract 1702) (see Figure 3). Benefit was observed in 7 out of 8 domains, including physical function, mental health, and bodily pain. The exception was general health, for which there was a numerical but not a statistically significant advantage for macitentan relative to placebo.
“It is important to evaluate HRQoL analyses in PAH trials in order to confirm a therapeutic benefit from the patient’s perspective,” reported Dr. Pavel Jana, Charles University, Prague, Czech Republic. “Although HRQoL was investigated as an exploratory end point in the SERAPHIN trial, the improvements provide additional reassurance that treatment is having a favorable impact.”
Figure 3.
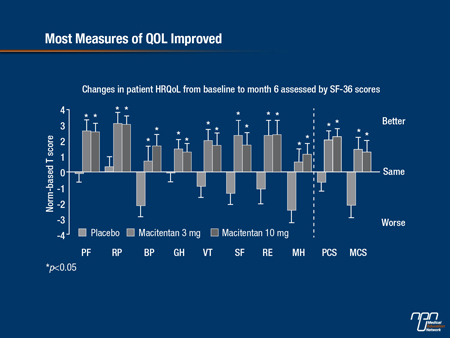
Conclusion
Data from large Phase III trials presented at the ERS predict fundamental changes in how the efficacy of treatments for PH is evaluated. In particular, the SERAPHIN trial, conducted with a novel ERA, demonstrated that effective treatments can improve outcomes as well as exercise capacity in PAH. In CTEPH, the CHEST-1 results demonstrated benefit with a first-in-class sGC stimulant on the basis of improved exercise capacity alone, but it was the first Phase III trial to show any benefit from a pharmacologic therapy in this type of PH. Both studies have the potential to serve as landmarks in the efforts to improve care for a set of disorders that, at present, are incurable.