Reports
Oral Biologics Gain Attention as Safety Data Catch Up with Efficacy Data in Rheumatoid Arthritis Control
This report is based on medical evidence presented at sanctioned medical congress, from peer reviewed literature or opinion provided by a qualified healthcare practitioner. The consumption of the information contained within this report is intended for qualified Canadian healthcare practitioners only.
MEDICAL FRONTIERS - 2012 Annual European Congress of Rheumatology (EULAR)
Berlin, Germany / June 6-9, 2012
Berlin - The potential to target the inflammatory cascade with oral rather than injectable targeted therapies in rheumatoid arthritis (RA) remains strong, based on studies evaluating agents with different mechanisms that were presented at the 2012 EULAR meeting. The 2 most promising targets for small-molecule agents so far have been the Janus kinase (JAK) and spleen tyrosine kinase (Syk) pathways. Inhibition of each has been associated with significant reductions in RA activity. The concern with these and all other immunomodulatory agents, including tumour necrosis factor (TNF) inhibitors—the first and still the most widely used targeted therapies in RA—is a potential increased vulnerability for infections or malignancies. This risk has proven to be low and acceptable with TNF inhibitors. If oral agents share the relative safety of the TNF inhibitors and other injectable biologics, the relative advantages for convenience and patient acceptance would be expected to be substantial.
Pathways to Reduced Disease Activity
Studies presented at the 2012 EULAR continue to generate encouraging data regarding the efficacy and safety of oral targeted therapies. New data were presented on a variety of agents, such as tofacitinib, a Janus kinase (JAK) inhibitor that has been tested extensively; GLPG0634, another JAK inhibitor in phase II trials; and fostamatinib, a spleen tyrosine kinase (Syk) pathway inhibitor. The ability of these agents to substantially reduce rheumatoid arthritis (RA) disease activity is no longer in doubt. The key issue is whether they are as safe and effective as injectable biologics. For this, each agent must be tested individually, but extended follow-up data becoming available for the most well studied oral agents have been reassuring.
Based on the data so far, “the new orally bioavailable kinase inhibitors will impact shortly on the strategies that we employ in the treatment of RA,” predicted Prof. Iain B. McInnes, University of Glasgow, UK. He indicated that it is still too soon to determine how the oral targeted therapies will fit with alternatives from the perspective of safety, efficacy and cost, but he does believe that newer oral targeted therapies will have utility in RA treatment, particularly if their efficacy is commensurate with injectable biologics.
Efficacy Data
So far, the data suggest that efficacy is comparable to injectable biologics. Patient outcomes data from a phase III randomized, double-blind trial that included a parallel treatment arm with adalimumab were presented at the 2012 EULAR. In the phase III study, 513 patients with an inadequate response to the non-biologic disease-modifying antirheumatic drug (DMARD) methotrexate (MTX) were randomized to oral tofacitinib 5 mg b.i.d., 10 mg b.i.d. or placebo. A parallel group of 204 patients were initiated at the same time on adalimumab 40 mg injected q2 weeks.
“Improvements from baseline in all 8 Short-Form 36 [quality of life] domains were statistically significantly better with tofacitinib 10 mg twice daily than placebo and numerically higher than adalimumab at 3 months before rescue of non-responders,” reported Prof. Ronald F. van Vollenhoven, Karolinska Institute, Stockholm, Sweden. After 3 months, when poor responders were switched to tofacitinib, the differences quickly diminished although there was still a significant advantage at 6 months (P<0.05) for those initially randomized to tofacitinib (Figure 1).
Figure 1.
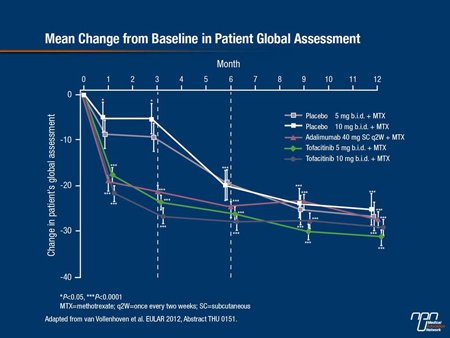
Tofacitinib has been evaluated in 5 phase III placebo-controlled, multicentre studies in RA, but this was the first to provide a side-by-side although non-randomized comparison to a tumour necrosis factor (TNF) inhibitor. In the study, all patients remained on MTX and on any other non-biologic DMARD they were taking in stable doses, such as nonsteroidal anti-inflammatory drugs (NSAIDs), opioids and/or low-dose oral corticosteroids.
At month 3, the proportions of patients reporting symptom improvement was 36% in the placebo group, 64% in the group receiving adalimumab, 68% of those receiving tofacitinib 10 mg and 70% of those receiving the 5-mg dose. After 3 months, placebo patients who had not achieved a 20% improvement in the American College of Rheumatology scale (ACR20) were switched to tofacitinib. At 12 months, those initiated on either dose of tofacitinib still did numerically better on several QOL scales, including physical functioning, than those initially randomized to placebo or adalimumab.
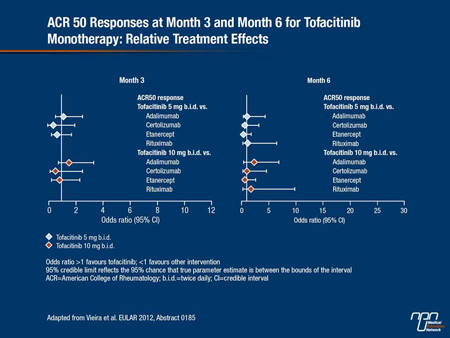
An indirect meta-analysis of efficacy studies presented at EULAR also provided support for a degree of efficacy with tofacitinib that is similar to injectable biologics. In this meta-analysis, 32 placebo-controlled trials were included. In addition to the studies with tofacitinib, these included studies with TNF inhibitors such as adalimumab, certolizumab and etanercept, as well as non-TNF inhibitor biologics such as abatacept and rituximab. All of the studies included patients who were not adequately controlled on MTX or other DMARDs. The agents were compared for the odds ratio for achieving ACR20, ACR50 and ACR70 responses. Exemplified by the ACR50 responses (Figure 2), the proportions at which any of the targeted therapies achieved responses (ACR20, ACR50 or ACR70) were similar at both 3 and 6 months.
“Whether evaluated as a monotherapy or in combination with MTX, tofacitinib provided responses that were comparable to the currently available TNF inhibitors. It was also at least as effective as other biologics, such as abatacept and rituximab,” reported Dr. Maria Cecilia Vieira, MAP Consultancy, Boston, Massachusetts. Although she cautioned that this type of analysis does not substitute for direct comparison due to the potential impact of such factors as the starting MTX dose and severity of disease, the data support a high rate of activity for tofacitinib. She also noted that “withdrawal rates due to adverse events [AEs] were comparable.”
Figure 2.
Safety in Daily Practice
The accumulating efficacy data with JAK inhibitors in RA which, in addition to tofacitinib, include ruxolitinib, baricitinib and GLPG0634, have provided compelling evidence that JAK is an appropriate target for the control of RA, but the safety data will be critical for moving these agents forward.
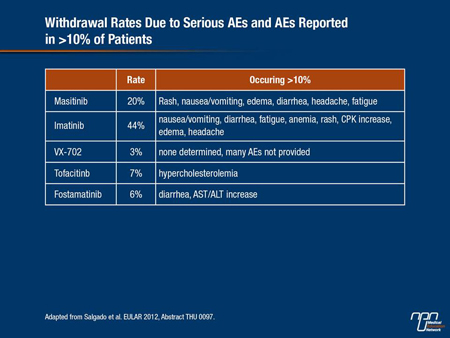
In a systematic review presented at EULAR on the safety of protein kinase inhibitors in RA, which included JAK inhibitors, Syk inhibitors and inhibitors of other targets, such as MAP kinase, 28 studies with >3000 participants were identified. While a broad range of AEs were associated with the various agents, the 2 drugs with the highest rate of withdrawal for serious AEs were masitinib (20%), a c-KIT inhibitor, and imatinib (44%), an ABL inhibitor. The lowest number of withdrawals was associated with VX-702 (3%), a MAP inhibitor, tofacitinib (7%) and the Syk inhibitor fostamatinib (6%), according to lead author Dr. Eva Salgado, Hospital Clínico Universitario de Santiago, Santiago de Compostela, Spain.
Table 1.
ACR Responses
There were no new clinical data presented on the JAK inhibitors ruxolitinib or baricitinib at EULAR, but results of a double-blind phase II study of GLPG0634 were presented by researcher Dr. Frédéric Vanhoutte, Galapagos NV, Mechelen, Belgium, where they are developing this agent. The study randomized 36 RA patients who had an inadequate response to MTX to GLPG0634
100 mg b.i.d., 200 mg once daily or placebo. The primary end point was the ACR20 response.
“This is the first JAK inhibitor selective for the JAK1 subtype,” Dr. Vanhoutte reported. “Overall, 83% of patients on active therapy vs. 33% of placebo patients [P<0.01] achieved the ACR20 response by 4 weeks. This remarkable result includes the absence of effects on LDL.” A dose-ranging study is now underway to advance clinical trials of this agent.
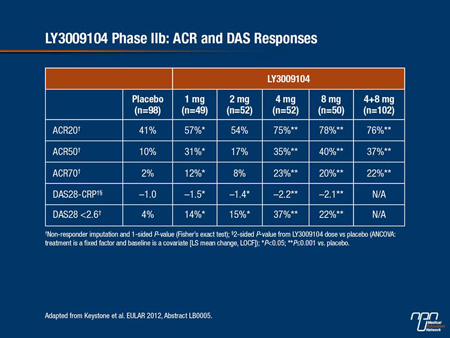
Double-blind data from a JAK1/JAK2 inhibitor, currently known as LY3009104, were also presented during the 2012 EULAR. In this phase IIb trial, 301 patients with active RA on stable doses of MTX were randomized to receive placebo or one of 4 once-daily doses of LY3009104, ranging from 1 mg to 8 mg. The study was conducted over 12 weeks.
“Onset of efficacy was rapid with statistically significant differences seen in the study end points from week 2 onwards,” reported Dr. Edward Keystone, Mount Sinai Hospital, Toronto, Ontario. The end points included ACR20, ACR50, ACR70 and the Disease Activity Score 28 (DAS28). At the 2 highest doses, 4 mg and 8 mg, 75% and 78%, respectively, achieved ACR20 vs. 41% of those randomized to placebo. ACR70 was reached by 23% and 20% of those receiving the 4-mg and 8-mg dose, respectively, and 2% of those receiving placebo.
Table 2.
Safety analyses associated LY3009104 with modest reductions in hemoglobin and modest increases in serum creatinine and LDL. The only opportunistic infection was observed in a patient in the placebo group. Overall, Dr. Keystone characterized this agent as well tolerated.
Significance of Targetting the JAK Pathway
The significance of specificity on JAK targets, such as JAK 1 or JAK1/JAK2, is still being explored, according to Dr. Joel Kremer, Albany Medical College, New York. Reviewing the JAK pathway at EULAR, Dr. Kremer described how JAK activation produces phosphorylation of intracytoplasmic STAT tyrosine kinases which in turn lead to nuclear transcription of inflammatory mediators. While it is clear that relative inhibition on the JAK1, JAK2 and JAK3 subtypes affect specific anti-inflammatory activity, the relevance to safety and efficacy in the management of RA is not yet well established.
“JAK inhibition in its various forms leads to downregulation of the interferon-related cytokines, the gamma-chain cytokines such as IL-2, IL-7, IL-9, IL-15 and IL-21, as well as the gp130 family, including IL-6 and related proteins,” Dr. Kremer explained. While JAK inhibition has been more recently “shown to inhibit TNF-induced release of chemokines such as IP-10, RANTES and MCP1,” the interrelated signalling complicates efforts to assign one JAK subtype to specific activities.
Exploring the Syk Pathway
The same principle of blocking enzymatic pathways important to cytokine release is being pursued for other targets, most notably Syk. There were limited new data on these agents at the 2012 EULAR meeting, but the safety of fostamatinib, one of the most well studied representatives of this class, was assessed in 2 EULAR studies. In one, the pharmacokinetics of fostamatinib was evaluated in patients with impaired hepatic function. This is relevant because hepatic metabolism plays an important role in the excretion of this agent. The other study, conducted by the same group of researchers, evaluated the effect of renal impairment on fostamatinib pharmacokinetics.
In the study of hepatic metabolism, 6 of the 24 participants had mild impairment of hepatic function, 6 had moderate impairment, 6 had severe impairment and 6 had normal hepatic function. Although a reduction in the maximum concentration and area-under-the-curve drug exposure correlated with increasing liver dysfunction, the authors reported that it did not reach a clinically relevant level. In the study of fostamatinib in renal impairment, 24 patients with varying degrees of impairment were also studied. Again, there was no clinically relevant reduction in drug exposure even with severe renal impairment. The authors concluded that these data suggest dose adjustments for hepatic or renal impairment are not likely to be needed.
In addition to the relative safety of immunomodulators, the introduction of oral small molecules designed to inhibit inflammatory pathways will intensify the search for biomarkers to predict responders. Currently, about 30% of RA patients have little or no response to TNF inhibitors. Some proportion of these patients will respond to an alternative agent from the same class, but the addition of new targets is likely to increase the number of patients who can be rescued with targeted therapies when their response to non-biologic DMARDs such as MTX is inadequate. If biomarkers can be developed, they may help guide patients to the immunomodulator most likely to be efficacious.
Summary
The new data presented on oral targeted therapies at the 2012 EULAR indicate that these therapies will soon expand options for the control of RA. A large array of agents with different targets are being pursued, but the studies with inhibitors of the JAK and Syk pathways have reached the most advanced stages of development. Phase III trials with tofacitinib continue to support the principle that intracellular mechanisms of the inflammatory cascade can be inhibited with acceptable safety and a high degree of inhibitory activity on RA activity. The long-term safety of these agents, like the long-term safety of all immunomodulators, must be demonstrated, but current data are encouraging.
Mednet reports accredited by McGill University under the MedPoint Accredited Conference Report Series are eligible for Mainpro-M1 and MOC Program credits. Please visit http://www.mednet.ca/en/credits.html to claim your Medpoint credits.