Reports
Rouge Valley Health System
This report is based on medical evidence presented at sanctioned medical congress, from peer reviewed literature or opinion provided by a qualified healthcare practitioner. The consumption of the information contained within this report is intended for qualified Canadian healthcare practitioners only.
PHYSICIAN PERSPECTIVE - Get With the Protocol - A Regional Perspective on the Evidence and Resulting Changes
August 2015
Guest Editor:
Saleem Kassam, MD
Department of Cardiology
Rouge Valley Health System
Toronto, Ontario
Introduction
In patients presenting with an acute coronary syndrome (ACS), early initiation of oral antiplatelet therapy is critical to the prevention of recurrent ischemic events. In a series of large trials, clopidogrel, prasugrel, and ticagrelor have all demonstrated protection against thrombotic events when combined with aspirin in ACS patients. These trials have also demonstrated that the therapeutic window, defined by the distance between a reduced risk of thrombus formation and an unacceptable increase in bleeding events, is narrow, differing for such variables as type of ACS, planned intervention, and time elapsed from onset of symptoms. Evidence-based guidelines, derived from the clinical trials, have been developed to lead to selection of the antiplatelet regimen most likely to provide an optimal benefit-to-risk ratio. At Rouge Valley Health System, an antiplatelet protocol for ACS patients has been adapted from current guidelines to accelerate the time to appropriate therapy.
Guidelines developed by the Canadian Cardiovascular Society (CCS) for the use of antiplatelet therapies in ACS patients have been developed and updated on the basis of large antiplatelet trials.1,2 These trials demonstrate that available regimens are not interchangeable for relative protection against thrombotic events or for their relative risk of adverse events, including increased risk of minor and major bleeding. The history of these trials traces the effort to improve antiplatelet efficacy to reduce risk of cardiovascular (CV) events without provoking an unacceptable rate of minor and major bleeding.
The modern era of dual antiplatelet therapy in ACS patients was initiated with the publication of the CURE trial in 2001.3 In CURE, patients with a non ST-segment elevated myocardial infarction (NSTEMI) who were randomized to receive clopidogrel in addition to aspirin had a 20% reduction (P<0.001) in the risk of a composite endpoint of ischemic events relative to those who received aspirin alone. A similar trial, CLARITY TIMI-28, showed an even greater relative risk reduction for clopidogrel when administered with aspirin relative to aspirin alone in patients with ST segment-elevated MI (STEMI).4
These trials demonstrated that greater platelet inhibition was associated with greater protection from CV events, but it was not clear whether still further risk reductions with acceptable safety were possible with more potent suppression of platelet activation, particularly among patients at highest risk. This was relevant not only to different types of ACS, a term that captures a spectrum of clinical conditions from unstable angina to no-flow thrombotic occlusions, but to ACS patients undergoing invasive procedures, such as percutaneous interventions (PCI), which can induce a counterproductive thrombotic response even as they are applied to restore and sustain blood flow.5
The series of antiplatelet ACS trials conducted since CURE and CLARITY TIMI-28, including those conducted with the newer agents prasugrel and ticagrelor, have established opportunities in which more effective platelet inhibition can provide further reductions in life-threatening CV events. The clinical trials have also yielded a new set of evidence to confirm a narrow gap between lower risk of CV events and greater risk of bleeding. In fact, event reductions from greater antiplatelet effect have not always been judged to warrant the increased risk of bleeding. Current guidelines are designed to identify how current options are best applied to achieve an optimal benefit-to-risk ratio on an evidence basis.
Targeting Platelet Activation
Relative to aspirin, which attenuates platelets by inhibiting cyclooxygenase and other enzymes that mediate activation,6 clopidogrel, prasugrel and ticagrelor inhibit P2Y12, a predominant activating receptor on the surface of the platelet.7 These P2Y12 inhibitors are not interchangeable. Both clopidogrel and prasugrel, which are thienopyridines, achieve inhibition of P2Y12 through a metabolite.8 For clopidogrel, a prodrug that is dependent on a two-step process mediated through the cytochrome P450 system, antiplatelet effect is delayed several hours after ingestion. Prasugrel is also converted into its active metabolite through hepatic metabolism but this is achieved in a single step. As a result the antiplatelet effect is achieved more quickly after ingestion of prasugrel than clopidogrel, and there appears to be less variability in response. Ticagrelor, a non-thienopyridine, is orally active with direct but reversible binding to the P2Y12 receptor.9 It also has a rapid onset of action and appears to provide a more predictable antiplatelet effect than clopidogrel.
The pharmacological differences between P2Y12 inhibitors have been shown to be clinically meaningful in large clinical trials. Although prasugrel and ticagrelor have not been directly compared, both agents have demonstrated greater protection against major CV events relative to clopidogrel when combined with aspirin in ACS populations. These multinational trials have been instrumental in developing the evidence-based CCS guidelines, which have now been adapted for use at Rouge Valley Health System.
The first challenge to the standard regimen of clopidogrel and aspirin in ACS patients was produced by the TRITON TIMI-38 trial.10 The trial compared prasugrel to clopidogrel in ACS patients scheduled for a PCI after coronary angiography was performed. More than 13,000 patients were randomized. All patients in both arms received aspirin plus an initial loading dose and then a maintenance dose of their assigned therapy. Approximately 75% of patients had NSTEMI and the remainder were STEMI ACS patients.
Relative to clopidogrel, prasugrel was associated with a 20% risk reduction (P<0.001) in the composite endpoint of death from CV causes, non-fatal myocardial infarction (MI), and non-fatal stroke. There were also significant reductions in a number of clinically meaningful endpoints, such as urgent target-vessel revascularization, stent thrombosis. However, there was a cost for prasugrel relative to clopidogrel in increased risk of major bleeding (P=0.03), life-threatening bleeding (P=0.01), and fatal bleeding (P=0.002). Overall mortality did not differ between the treatment arms.
In TRITON TIMI-38, the absolute reduction in risk of CV events exceeded the absolute increase in bleeding, but an effort to improve the benefit-to-risk ratio prompted a series of subsequent analyses that revealed relative bleeding risk to be higher in older patients, patients with a history of stroke or prior transient ischemic attack (TIA), and patients with low body weight.
A second trial called PLATO evaluated ticagrelor in a much broader ACS population.11 In that trial, more than 18,000 patients admitted to a hospital for ACS without regard to the planned intervention or pre-hospital antiplatelet therapy were randomized to ticagrelor or clopidogrel. Again, all patients received aspirin and were initiated on their assigned therapy with a loading dose followed by a maintenance regimen. The NSTEMI proportion of the enrolment was slightly lower in PLATO than TRITON TIMI-38 at 63%.
Relative to clopidogrel, ticagrelor was associated with a 16% reduction (P<0.001) in the risk of the same endpoint as that used in TRITON TIMI-38. Significant reductions in secondary endpoints favouring ticagrelor included death from CV causes (P=0.005). In addition, ticagrelor was associated with a significant reduction in all-cause mortality (P<0.001). One explanation for this additional advantage is that ticagrelor was associated with a much lower propensity for major bleeding in PLATO than prasugrel in TRITON TIMI-38. Overall, major bleeding rates did not differ significantly between the ticagrelor and clopidogrel arms. Major bleeding not related to coronary artery bypass grafting (CABG) was higher in the ticagrelor arm, but rates of fatal bleeding were not significantly different.
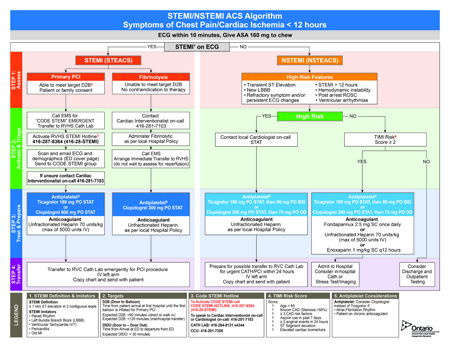
Both TRITON TIMI-38 and PLATO demonstrate that greater CV risk reductions can be achieved with newer antiplatelet agents relative to clopidogrel when combined with aspirin. While greater antiplatelet effect may be important, speed of onset may also play a role. It is noteworthy that double-the-dose versus standard-dose clopidogrel did not provide an advantage for CV endpoints in the CURRENT OASIS-7 trial overall,12 although a 14% reduction (P=0.039) in a composite endpoint of CV events was achieved by the higher dose in the subpopulation of PCI patients.13 CURRENT OASIS-7 and a previous study, PCI-CURE,14 also found no advantage but increased risk for high-dose aspirin.
Antiplatelet ACS Guidelines
The large antiplatelet trials conducted over the past 15 years have informed treatment guidelines, identifying opportunities where risk of CV events can be lowered with a net benefit relative to the potential for increased bleeding. These trials demonstrate that antiplatelet therapies are not interchangeable, particularly in patients at high risk of a CV event. In the most recent CCS guidelines, four algorithms have been provided to address different levels of thrombotic risk in ACS populations. These are NSTEMI patients receiving immediate antiplatelet therapy, NSTEMI patients with a planned PCI who have not yet received a P2Y12 inhibitor, STEMI patients, and ACS patients undergoing CABG.
When combined with aspirin, ticagrelor or prasugrel are preferred over clopidogrel in most pathways outlined in the CCS guidelines, but there are exceptions. This includes STEMI patients who do not proceed to PCI and are managed with fibrinolytic therapy. In this group, clopidogrel is preferred due to concern over increased risk of bleeding and lack of trial data showing an advantage for newer agents. Clopidogrel is also the preferred antiplatelet partner with aspirin in patients undergoing CABG. When prasugrel is an option, such as in NSTEMI patients with a planned PCI, the guidelines recommend a reduced dose in older patients and patients weighing <60 kg. A history of stroke or TIA is a contraindication for prasugrel. Antiplatelet therapy is maintained for 12 months after the ACS admission.
At Rouge Valley Health System, these guidelines have been adapted into a STEMI/NSTEMI algorithm that is designed to streamline decisions for time-sensitive care. These focus on pathways most likely to rapidly restore or sustain perfusion while minimizing bleeding risk. In an effort to achieve the greatest benefit-to-risk ratio of care, complex decision trees are avoided. In NSTEMI patients, for example, clopidogrel is preserved as a treatment option even in those with high-risk features because it is readily available and may be the most expedient choice. In the PLATO study, ticagrelor did show superiority in this group of patients, but dual antiplatelet therapy with clopidogrel does reduce risk of CV events and remains an acceptable choice. Prasugrel has not been included in the algorithm even in STEMI patients eligible for PCI because of relative contraindications, such as prior stroke, that complicate a simple decision tree.
In all settings, clear pathways of intervention can be useful in developing reproducible and rapid quality of care. In regional hospitals, such as Rouge Valley, an effort to adapt decision-making to the available resources and expertise has been undertaken within an evidence-based context. In the STEMI/NSTEMI algorithm, the specific recommendations for antiplatelet therapy are evidence-based but practical, incorporating the concept that the optimal benefit-to-risk ratio will be derived from choices associated with low risk of increased bleeding and can be implemented rapidly.
Conclusion
Large clinical trials demonstrate that currently available antiplatelet therapies are not interchangeable. Relative to clopidogrel, both ticagrelor and prasugrel have demonstrated a reduced risk of ischemic events in selected ACS populations. These data are useful but require translation into practical patient management pathways that are clear and sufficiently simple to minimize delays. The guidelines developed at Rouge Valley have been adapted from the CCS guidelines in an effort to introduce opportunities for improved outcome in the context of local practice.
Questions & Answers
Q: Now that there is an algorithm that expands choices for dual antiplatelet therapy, are you concerned that these decisions will delay care?
Dr. Kassam: No; I think the absence of an algorithm leads to delays where a physician is unsure of the ‘right’ approach. Pathways streamline care and reinforce that either antiplatelet choice is acceptable. I don’t think this will lead to delays.
Q: Do you feel your treatment algorithm for dual antiplatelet therapy is reassuring for the clinician who fears causing a bleeding event more than failing to prevent a recurrent thrombotic event?
Dr. Kassam: Yes, as it explicitly doesn’t mention contra-indications up front, and because it provides an opportunity for discussion with the specialist as needed.
Q: In patients scheduled for a PCI, prasugrel was superior to clopidogrel in TRITON TIMI-38. You do not single out this group in your algorithm. Why?
Dr. Kassam: We didn’t want to introduce too many qualifiers for which prasugrel should or should not be given. Where it has been dominant, prasugrel has not been compared directly with ticagrelor. Finally, keeping the pathways simple will lead to expedient treatment and better health care worker familiarity and ease-of-use.
Q: Do you think that a subset of high-risk patients may be eventually identified who would benefit from regimens with even greater antiplatelet activity?
Dr. Kassam: I think we may be approaching a point of diminishing returns, and greater risks, with stronger agents. I see value in being able to inhibit and reverse platelet reactivity faster, as a potential therapeutic benefit.
References
1. Bell AD, Roussin A, Cartier R, et al. The use of antiplatelet therapy in the outpatient setting: Canadian Cardiovascular Society guidelines. Canadian Journal of Cardiology 2011;27 Suppl A:S1-59.
2. Tanguay JF, Bell AD, Ackman ML, et al. Focused 2012 update of the Canadian Cardiovascular Society guidelines for the use of antiplatelet therapy. Canadian Journal of Cardiology 2013;29:1334-45.
3. Yusuf S, Zhao F, Mehta SR, et al. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation.
N Engl J Med 2001;345:494-502.
4. Sabatine MS, Cannon CP, Gibson CM, et al. Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST-segment elevation. N Engl J Med 2005;352:1179-89.
5. Fuster V, Falk E, Fallon JT, Badimon L, Chesebro JH, Badimon JJ. The three processes leading to post PTCA restenosis: dependence on the lesion substrate. Thrombosis and Haemostasis 1995;74:552-9.
6. Awtry EH, Loscalzo J. Aspirin. Circulation 2000;101:1206-18.
7. Damman P, Woudstra P, Kuijt WJ, de Winter RJ, James SK. P2Y12 platelet inhibition in clinical practice. Journal of Thrombosis and Thrombolysis 2012;33:143-53.
8. Wiviott SD, Antman EM, Braunwald E. Prasugrel. Circulation 2010;122:394-403.
9. Nawarskas JJ, Clark SM. Ticagrelor: A novel reversible oral antiplatelet agent. Cardiology in Review 2011;19:95-100.
10. Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007;357:2001-15.
11. Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2009;361:1045-57.
12. Investigators C-O, Mehta SR, Bassand JP, et al. Dose comparisons of clopidogrel and aspirin in acute coronary syndromes. N Engl J Med 2010;363:930-42.
13. Mehta SR, Tanguay JF, Eikelboom JW, et al. Double-dose versus standard-dose clopidogrel and high-dose versus low-dose aspirin in individuals undergoing percutaneous coronary intervention for acute coronary syndromes (CURRENT-OASIS 7): A randomised factorial trial. Lancet 2010;376:1233-43.
14. Jolly SS, Pogue J, Haladyn K, et al. Effects of aspirin dose on ischaemic events and bleeding after percutaneous coronary intervention: insights from the PCI-CURE study. European Heart Journal 2009;30:900-7.