Reports
Targeted Therapies Providing Major Clinical Advances in Myeloproliferative Neoplasms
This report is based on medical evidence presented at sanctioned medical congress, from peer reviewed literature or opinion provided by a qualified healthcare practitioner. The consumption of the information contained within this report is intended for qualified Canadian healthcare practitioners only.
MEDICAL FRONTIERS - 53rd Annual Meeting & Exposition of the American Society of Hematology
San Diego, California / December 10-13, 2011
San Diego - Progress in understanding the molecular pathophysiology of myeloproliferative neoplasms (MPNs) is generating new therapies that appear capable of inhibiting key steps in the disease process. In myelofibrosis, polycythemia vera and essential thrombocythemia, Janus kinase (JAK) has been isolated as an important signalling pathway and an attractive target for disease control. Although a variety of other therapies, including immunomodulating agents, are also being pursued, the potential benefits of inhibiting a central mediator of MPN expression has accelerated testing of several JAK inhibitors, including one that was evaluated in 2 simultaneous phase III trials. The clinical studies to date have not been powered to demonstrate a survival benefit but the symptomatic benefits have been substantial.
Chief Medical Editor: Dr. Léna Coïc, Montréal, Quebec
Myeloproliferative neoplasms (MPNs), which include myelofibrosis (MF), polycythemia vera (PV), essential thrombocythemia (ET), chronic myeloid leukemia, chronic neutrophilic leukemia, chronic eosinophilic leukemia and mast cell disease, develop when a defect of the myeloid lineage accelerates hematopoiesis, leading to fibrosis in the bone marrow, overproduction of red blood cells and production of dysfunctional platelets. All can result in splenomegaly, an increased risk of thrombosis and debilitating symptoms. Janus kinase (JAK) mutations isolated in MF, PV and ET disorders range from 50% in MF and ET to 95% in PV. Dysregulation of JAK function can occur in the absence of mutations for a number of reasons such as alterations of the activation loop in the catalytic domain.
Due to the emerging data that suggest JAK inhibitors offer benefit across several MPN subtypes, including those without a JAK mutation, targeted inhibition of JAK-STAT function appears to be important. The majority of primary and secondary MF, PV and ET neoplasms are characterized by a V617F mutation which produces a gain of function in JAK2 signalling. “While this provides an important target for therapy, we also know that wild-type JAK2 plays a pivotal role in normal hematopoiesis. It is therefore potentially important to inhibit JAK2 but to do so selectively in order not to impede normal marrow function,” explained Dr. Srdan Verstovsek, Department of Leukemia, M.D. Anderson Cancer Center, University of Texas, Houston.
In addition to MPN, the JAK signalling pathway has been found to be a promising target for a number of proliferative processes, including cancer, and autoimmune activity in inflammatory diseases such as rheumatoid arthritis. As a result, there are several JAK inhibitors in development for clinical application.
COMFORT Studies
The phase III data that provided the basis of the recent FDA approval for a JAK inhibitor in MF were first presented at the 2011 meeting of the American Society of Clinical Oncology (ASCO) and updated here at the 2011 ASH. The agent, ruxolitinib, was evaluated in 2 simultaneously conducted trials called COMFORT-I and COMFORT-II.
“The most significant finding from the recent analyses of the COMFORT studies is the consistency of effect across the subgroups. The relative benefit of ruxolitinib over best available therapy [BAT] was observed in all of the neoplasm types, whether or not the V617F mutation was present, in patients older and younger than 65 years of age, and regardless of stratification by spleen volume,” reported
Dr. Claire N. Harrison, Department of Haematology, Guy’s and St. Thomas NHS Foundation Trust, London, UK (Figure 1).
According to Dr. Harrison, investigator-defined BAT could include hydroxyurea and treatments for anemia, such as low-dose prednisone, erythropoiesis-stimulating agents or androgens.
Figure 1.
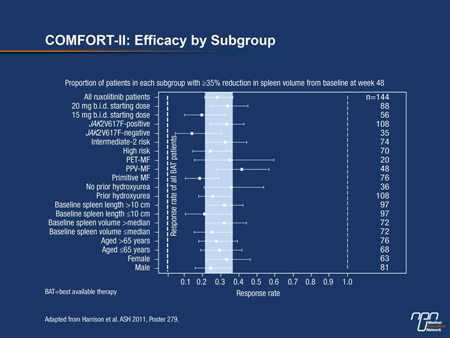
Of similar design, the 2 COMFORT studies enrolled patients with intermediate- or high-risk MF, post-PV MF (PPV-MF) or post-ET MF (PET-MF). In COMFORT-I, which included centres in Canada, 309 patients were randomized to the oral agent ruxolitinib 15 mg or 20 mg b.i.d. depending on platelet count or to placebo. In COMFORT-II, which was conducted in Europe, 219 patients were randomized to the same doses and schedule of the JAK2 inhibitor based on platelet level or to BAT, which could include hydroxyurea, blood transfusions, steroids, allopurinol and other therapies often employed in supportive care.
The results of the 2 trials were mutually reinforcing. After 24 weeks of therapy in COMFORT-I, 42% of the patients had a reduction in spleen volume ≥35% vs. 1% of those randomized to placebo (P<0.0001). In COMFORT-II,
by the end of 24 weeks of treatment, the proportions were 31.9% and 0% for active treatment and BAT, respectively (P<0.0001). The proportion of patients with ≥50% improvement in the total symptom score (TSS) in COMFORT-I, which included abdominal discomfort, early satiety, itching, night sweats and bone pain, also favoured ruxolitinib (45.9% vs. 5.3%; P<0.0001). Although the COMFORT studies
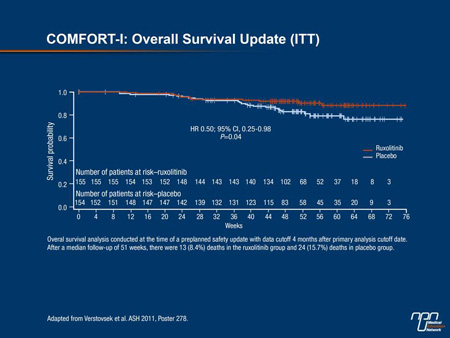
were not powered for survival, in COMFORT-I, the hazard ratio for survival was improved 50% with JAK inhibition (HR 0.50; 95% CI, 0.25-0.98; P=0.04) (Figure 2).
Both studies reported large improvements in quality of life (QoL). “The reduction in the spleen volume was dramatic in many patients, but we were particularly impressed with the symptomatic response. Patients with quite debilitating symptoms achieved substantial relief of their symptoms, and this has not been seen with other therapies,” Dr. Harrison reported.
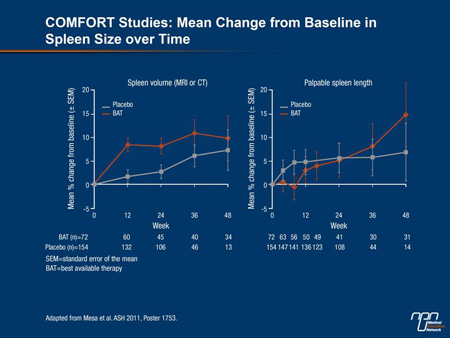
A post-hoc analysis of the COMFORT studies provided more detail about the relative importance of an effective treatment for MF. There were no differences in outcome in COMFORT-II patients receiving BAT and those who received placebo in COMFORT-I, according to Dr. Ruben Mesa, Mayo Clinic, Scottsdale, Arizona (Figure 3). Rather, “neither had clinically meaningful improvements from baseline in any of the QoL or symptom scores,” and the increase in spleen size was “numerically similar.” In effect, the data suggest that BAT before the introduction of ruxolitinib provided “very little improvement in spleen size, symptoms or QoL.”
Figure 3.
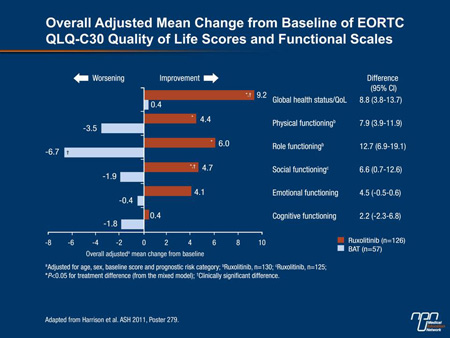
A rigorous QoL analysis was part of the design of COMFORT-II and included regular assessments throughout the course of the study with the EORTC QLQ-C30 and the FACT-Lym questionnaires. The EORTC QLQ-C30 includes 5 functional scales and 9 symptom scales. FACT-Lym is designed to evaluate 4 domains of well-being, including those that capture changes in emotional and social status. While these scales often showed worsening of QoL on BAT, the consistency of improvement on ruxolitinib was not only statistically significant but also “clinically important,” according to Dr. Harrison (Figure 4).
Figure 4.
Supportive Data for JAK2 Inhibition
The benefit of JAK2 inhibition observed in the COMFORT trials even in patients without the characteristic V617F mutation validates the JAK2 pathway as a key mediator of disease and a critical target for therapy. It is not surprising, therefore, that other JAK2 inhibitors are in development. Clinical experiences with several agents, including a phase II trial with pacritinib, were presented at the 2011 ASH meeting. Overall, these provided additional support for the activity of JAK2 inhibition against these diseases. In the uncontrolled phase II study of pacritinib, 34 patients with MF and splenomegaly were started on 400 mg daily and followed primarily for change in spleen size and safety.
“Almost 90% showed reductions in palpable splenomegaly, while 44% showed decreases of ≥50% and 18% achieved clinical resolution of splenomegaly,” reported Dr. Rami S. Komrokji, Moffitt Cancer Center, Tampa, Florida. Notably, spleen size reductions were comparable in those with a low platelet count when compared to those with normal platelet levels. At 6 months of follow-up, spleen size reductions correlated with a significant reduction in MF symptoms, including abdominal pain, bone pain and fatigue. While the most common side effects were gastrointestinal, Dr. Komrokji characterized these as being of low grade and manageable.
“Pacritinib shows promising efficacy in alleviating MF-associated splenomegaly and constitutional symptoms at a dose that induces minimal myelosuppression,” he added, alluding to a potential concern of JAK2 inhibition that has not been a clinical issue in trials so far. “Given the lack of myelosuppression with pacritinib, this JAK2 inhibitor is of particular importance for MF patients with impaired hematopoiesis.”
Promising Strategies in MPN
However, JAK2 inhibitors are not the only treatments with promise in MPN. Several sets of new data were presented with immunomodulators, histone deacetylase (HDAC) inhibitors and pegylated interferon alpha-2a. In a summary of 3 consecutive trials with the immunomodulator pomalidomide, data on 82 patients were available of which 63 had been evaluated in a phase III study. All patients were dependent on red blood cell transfusions at entry. Although only 22 (27%) met predefined response criteria for relief of anemia, 21 (96%) of the responders had improved within several months of starting therapy.
The 2 best predictors of response were presence of JAKV617F mutation and the absence of splenomegaly, according to Dr. Kebede Begna, Mayo Clinic, Rochester, New York. Although peripheral neuropathy may be an obstacle for long-term treatment, some patients were able to remain on pomalidomide for up to 36 months.
The HDAC inhibitor givinostat inhibits cells that contain the JAKV617F mutation. It was tested in 44 patients with PV who were non-responders to the maximally tolerated dose of hydroxyurea. In the phase II study, patients remained on hydroxyurea and were randomized to receive givinostat 50 mg or 100 mg. Improvement in symptoms, which did not differ markedly between groups, was observed in 95% of patients. The therapy was particularly effective in the control of itching, according to Dr. Guido Finazzi, Ospedali Riuniti, Bergamo, Italy. He noted that all treatment-related side effects, including diarrhea and thrombocytopenia, were grade 2 or milder and suggested that further development is warranted, particularly in patients with refractory itching.
Several studies with pegylated interferon alpha-2a in MPN were presented including one that collated the experience at several centres. In this study, response rates were evaluated in 115 patients retrospectively. Of these, 54 (47%) had PV, 44 (38%) had ET and 17 (15%) had MF. The majority had received at least 1 prior treatment, including hydroxyurea, anagrelide, ASA, a non-pegylated interferon or phlebotomy. The median starting dose was 45 g (22.5g to 100 g).
The activity rates were high in all 3 MPN subtypes with about half of PV and ET patients achieving a complete response. Both hematologic and non-hematologic toxicities were generally mild, according to the senior author,
Dr. Krisstina Gowin, Keck School of Medicine, University of Southern California, Los Angeles. Although 17% of patients discontinued therapy due to toxicities, therapy was characterized as “well tolerated,” particularly in the context of the activity against MPN symptoms. “Given the majority of patients had previously failed cytoreductive therapy, these results substantiate prior reports of efficacy of pegylated interferon alpha-2a in MPNs,” Dr. Gowin stated.
Summary
Progress in the treatment of MPN includes a phase III study with a JAK inhibitor that provided substantial reductions in spleen size and impressive symptom reductions. The trial has already provided the basis for US regulatory approval for the agent in the treatment of MF. Additional JAK inhibitors in development and treatments with other mechanisms of action may further expand therapeutic options for these diseases. The activity in this area is highly encouraging because of the limited therapeutic options that have been available in the past.