Reports
PNH in the Pandemic: New Science and New Treatments in the Spotlight at ASH 2021
This report is based on medical evidence presented at sanctioned medical congress, from peer reviewed literature or opinion provided by a qualified healthcare practitioner. The consumption of the information contained within this report is intended for qualified Canadian healthcare practitioners only.
PRIORITY PRESS - 63rd Annual Meeting of the American Society of Hematology (ASH)
In-person/virtual, Atlanta, Georgia / December 11–13, 2021
In-person/Virtual – The 63rd ASH Annual Meeting featured a solid scientific line-up on paroxysmal nocturnal hemoglobinuria (PNH), despite the rarity of the disease, with five events and 23 posters. For Canadian PNH specialists, the key data surrounded C5-inhibitor ravulizumab, the second therapy available in Canada for PNH after an 8-year gap which made its appearance during the COVID-19 pandemic. Also at the meeting were reality checks on pivotal-trial results through the power of registry and EMR data, correlations between clinical parameters and patient quality-of-life (QoL) and timely clinical guidance on SARS-CoV-2 vaccination in PNH patients.
Chief Medical Editor: Dr. Léna Coïc, Montréal, Quebec
“[These are] really exciting times in complement therapeutics,” said Dr. Spero Cataland, professor in the Division of Hematology at Ohio State University Medical Center. “The most exciting thing is the emerging understanding that complement activation plays a part (minor or major) in many diseases.”
Dr. Cataland chaired a satellite symposium at the 63rd ASH Annual Meeting on the evolving landscape of complement inhibition therapy in diseases such as (PNH).1
PNH is a rare, chronic and life-threatening disease in which uncontrolled complement activation leads to thrombosis, organ damage, intravascular hemolysis and fatigue, dyspnea and pain. Although rare, PNH has triggered research pathways that could have wider benefits.
Dr. Cataland, interviewed following the symposium, said that the increasing number of complement-directed therapeutics, “will without question lead to new treatment for numerous diseases going forward.”
Broadening Therapeutic Options in Canada
Until this Fall, C5-inhibitor eculizumab, approved by Health Canada in 2009, was the sole Canadian treatment for PNH. It is now joined by ravulizumab, a new C5 inhibitor with a longer half-life and a less frequent dosing interval than eculizumab, indicated in patients ≥ 18 years.
In a product theater at the conference2, Dr. Anita Hill from Alexion reviewed key data for ravulizumab, which can be dosed at 8-week intervals in contrast to eculizumab’s biweekly dosing. The pivotal data for ravulizumab come from two adult studies, one each in naïve and switch patients, and a pediatric study.3–7
The adult inhibitor-naïve study compared ravulizumab to eculizumab in a 26-week randomization phase and a ravulizumab-only 26-week extension (N=246).3–5 Ravulizumab was non-inferior to eculizumab over the 26 weeks’ randomization for all endpoints: normalization of LDH (lactate dehydrogenase; 53.6% vs. 49.4%), transfusion avoidance (73.6% vs 66.1%) and Hb stabilization (68.0% vs. 64.5%). During 1 year of ravulizumab treatment 95% of patients avoided breakthrough hemolysis and 98% were free of major adverse vascular events (MAVE).
Ravulizumab’s ‘switch’ study randomized patients stable on eculizumab to either ravulizumab (n=97) or continued eculizumab (n=98) for 26 weeks, after which all patients received ravulizumab.3,4,7
Again, ravulizumab was non-inferior to eculizumab across all endpoints, said Dr. Hill. In the randomization phase 87.6% of ravulizumab patients avoided transfusion vs 82.7% of eculizumab patients and had zero breakthrough hemolysis or MAVE.
Data for pegcetacoplan, a C3 inhibitor recently approved in the U.S., appeared in a poster from Dr. Antonio Risitano, a hematologist from the University of Naples, Italy, and colleagues.8 (Although the drug is not yet approved in Canada, a pegcetacoplan trial is currently running at the University of Calgary and Toronto General Hospital [NCT03531255]).
Dr. Risitano’s team presented a post-hoc analysis of data from phase 1b, phase 2b and phase 3 trials analyzing the percentage of patients with a hematologic response to pegcetacoplan at week 48. Between 54% and 63% of patients had a good/major/complete hematological response to pegcetacoplan at week 48 (62/104 patients across all three trials).
Real-world C5 Inhibitor Data
Dr. Louis Terriou of the University of Lille, France, presented a poster on long-term survival for eculizumab, drawing on the power of the 14-year accumulation of data in the International PNH Registry.9
The study rationale was that, “previous analyses…leveraged historical data and were limited by small patient numbers and short follow-up durations.”
Dr. Terriou’s team compared survival for 1,892 patients who had received eculizumab for at least 35 days (‘ever-treated’ patients) vs 2,735 ‘never-treated’ patients. At 20 years, overall survival for ever-treated patients was 82%, versus 69% in people who did not get the drug. Overall hazard ratio for death in the ever-treated patients was 0.48 (95% CI, 0.39–0.60; p<0.0001), a 52% increase in survival for patients treated with eculizumab. This trend held when the data were stratified by disease activity at baseline (Figure 1).
Figure 1.
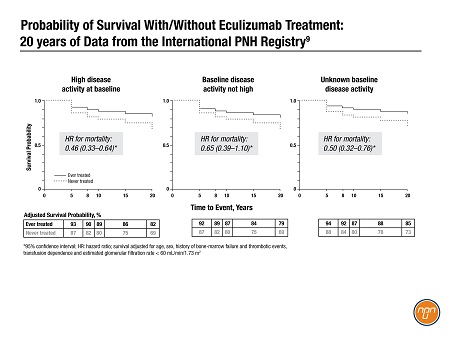
The authors concluded, “Survival benefits conferred by eculizumab treatment were observed regardless of [disease activity] status at baseline…and were maintained through 2 decades of real-world follow up.”
A poster headed by Dr. Michael Yeh of Apellis Pharmaceuticals analyzed real-world data for 182 PNH patients in a U.S. EMR network captured 2010–2021.10 The researchers found that Hb had stabilized within 6 months in 95% of the patients on ravulizumab and 93% of those on eculizumab. There was little change in Hb when patients switched drugs; the authors attributed this to the drugs’ identical mechanisms of action.
Real-life Fatigue and Life Quality
The International PNH Registry also yielded a poster at ASH on patient-reported outcomes (PROs). Dr. David Cella of the Northwestern University Feinberg School of Medicine looked at real-world experience of fatigue in patients on eculizumab11, seeking a comparison with the pivotal-trial evidence. He and his colleagues from academia and Alexion AstraZeneca pulled together FACIT-Fatigue scores at baseline and after 6, 12, 24 and 36 months on eculizumab (N=423).
At baseline, 93% of the patients reported fatigue, with a mean FACIT-Fatigue score of 29.4. At all time points thereafter, approximately two-thirds of patients had one or more clinically important differences in their fatigue scores.
The authors said that their results didn’t match the numerical improvement seen in eculizumab’s pivotal study TRIUMPH; however, “This finding, obtained from a real-world dataset with a large number of patients, helps establish an important metric for assessment of the meaningful treatment response of patients with PNH,” they said.
The conference also covered PROs for ravulizumab – fatigue and QoL. Dr. Hubert Schrezenmeier of the University of Ulm, Germany, found that patient fatigue and QoL in ravulizumab’s pivotal trial in treatment-naive patients were significantly correlated with LDH levels, but not Hb – until the LDH level reached or fell below 1.5 x ULN at day 183.12 This “[highlights] the importance of controlling intravascular hemolysis in patients with PNH,” said the author.
COVID-19 Vaccination
One poster during this pandemic-era meeting covered SARS-CoV-2 vaccination for patients with PNH. A multicentre team from Italy headed by Dr. Juri Alessandro Giannotta of the Policlinico of Milan conducted a survey of five Italian centres whose PNH patients had completed the COVID-19 vaccination schedule (N=67).13 In total, 6% of the patients had a PNH-related exacerbation – one hemolytic exacerbation and three breakthrough hemolyses. Three of the episodes occurred within 24–48 hrs of the second dose. The Italian team concluded that the flares were “clinically relevant but manageable and should not discourage vaccination.”
Conclusions
In Canada, patients and physicians now have a second therapeutic option, ravulizumab, with non-inferior data but easier dosing than incumbent, eculizumab. Real-world evidence from registries and EMRs adds valuable clinical information to pivotal trial data in this rare disease. Patients with PNH who ‘flare’ after SARS-CoV-2 vaccination can be managed.
References:
1. Cataland S, et al. The evolving landscape of complement inhibition therapy: implications for hematology practice. Satellite symposium at 63rd ASH Annual Meeting, Dec 10, 2021.
2. Hill A. Importance of sustained terminal complement inhibition in PNH. Product theater at 63rd ASH Annual Meeting, Dec 12, 2021.
3. www.ultomirishcp.com. Data on file. Accessed Dec 21, 2021.
4. Ultomiris product monograph. Sept 22, 2021.
5. Lee JW, et al. Blood 2019; 133:530–539.
6. Schrezenmeier H, et al. Ther Adv Hematol. 2020;11:2040620720966137.
7. Kulasekararaj AG, et al. Blood 2019; 133:540–549.
8. Risitano A, et al. Poster 1104, ibid.
9. Terriou L, et al. Poster 2188, ibid.
10. Yeh M, et al. Poster 1112, ibid.
11. Cella D, et al. Poster 1952, ibid.
12. Schrezenmeier H, et al. Poster 2196, ibid.
13. Giannotta J, et al. Poster 2180, ibid.