Reports
Complement in the kidney and beyond: Understanding pathophysiology and treatment outcomes in aHUS
This report is based on medical evidence presented at sanctioned medical congress, from peer reviewed literature or opinion provided by a qualified healthcare practitioner. The consumption of the information contained within this report is intended for qualified Canadian healthcare practitioners only.
PRIORITY PRESS - Kidney Week 2023: Annual Meeting of the American Society of Nephrology
Philadelphia, Pennsylvania / November 2–5, 2023
Philadelphia – Atypical hemolytic uremic syndrome (aHUS) is a thrombotic microangiopathy (TMA) in which dysregulation of the complement cascade leads to microvascular thrombi, microangiopathic hemolytic anemia, thrombocytopenia, and damage to the kidneys and/or other end organs. Although it is considered a rare disease, a number of presentations and posters at the recent Kidney Week conference provided new insights into aHUS management, and gave a glimpse of how our evolving understanding of complement regulation and dysregulation, and the potential role of complement in a constellation of interconnected diseases and conditions, may eventually help us offer more personalized and comprehensive patient care.
Chief Medical Editor: Dr. Léna Coïc, Montréal, Quebec
Atypical HUS is a complement-mediated disease with primarily renal clinical manifestations. It is designated as “atypical” to distinguish it from the more common form of hemolytic uremic syndrome, which is generally precipitated by an infection by Shiga-toxin-producing E. coli bacteria (STEC-HUS). In aHUS, patients with an inherited or acquired predisposition to complement dysregulation develop overt TMA, often after a triggering event that damages the vascular endothelium.
Although aHUS has traditionally been considered to be a rare disease that mainly affects the kidney, new data presented at Kidney Week may help expand our view of both the incidence of aHUS and its interconnection with complement-mediated symptoms in other body systems. Dr Sara Boynton, Johns Hopkins Children’s Center, Baltimore, and colleagues investigated the incidence of aHUS in over 20,000 pediatric patients with chronic or end-stage kidney disease enrolled in the North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS) Registry. They identified a subset of patients who were eligible for transplant who did not have a prior aHUS diagnosis, but whose renal pathology was either of unknown etiology or linked to conditions associated with TMA. Of the 95 patients included in the analysis, about one-third were diagnosed with one or more markers of clinical TMA, with 42% exhibiting both thrombocytopenia and microangiopathic hemolysis in addition to their renal symptoms. Extrarenal manifestations of TMA were also common: hypertension was present in 31% of individuals, while 19% had gastrointestinal symptoms and 44% had growth failure or were underweight. “This analysis shows that there might be a higher prevalence of aHUS in the NAPRTCS registry than previously thought,” they concluded.1
To understand the implications for Canadian clinicians of these and other data presented at Kidney Week, Medical Education Network interviewed Dr Christoph Licht of SickKids Hospital, Toronto, an international expert on complement-mediated kidney diseases and a key contributor to the Global aHUS Registry. “The extrarenal manifestations [of aHUS] basically speak to the fact that complement has a much broader pathology and is not limited to the kidneys,” said Dr Licht. “We need to consider it in a much broader sense when we think about unexplained disease. And, of course, it always comes with the treatability - we have specific drugs that allow for intervention at a point where we didn't have anything specific to offer in the past.”
Dr Licht said that one of the most intriguing sessions for him at Kidney Week was a presentation by Dr Richard Coward, University of Bristol, England, and colleagues, that posed “provocative” questions about a potential role for complement in recurrent disease in the kidneys and elsewhere. “That was maybe the biggest ‘splash’ at the meeting for me: the talk dared to say something that we’re doing some work on and others have thought about, but now it was being said on stage – could it be that we completely underestimate the relevance and the quantity of complement-mediated conditions that lead to pathologies that we call something else, but the underlying defect really is complement?”2
Insights into real-world outcomes with C5 inhibitors
Eculizumab and ravulizumab are intravenously administered inhibitors of the terminal complement component C5 that are indicated in Canada for management of aHUS in adults and in children aged 1 month and older. Eculizumab, with its two-week dosing interval, was first to market and became established as the standard of care; ravulizumab, a next-generation product based on eculizumab with an eight-week dosing schedule, has become available in many countries over the past few years. Several posters at Kidney Week used real-world data to describe how having two treatment options has affected clinical and patient-reported outcomes.
Dr Franz Schaefer, Heidelberg University Hospital, Germany, and colleagues described patient characteristics and clinical outcomes for 43 adults and 17 children switching from eculizumab to ravulizumab who were part of the Global aHUS Registry, a multinational collaboration that has been collecting real-world data on aHUS outcomes since 2012. About one-third of patients had previously undergone kidney transplantation, and extra-renal involvement was common, notably in the gastrointestinal system (48%) and central nervous system (32%). Overall, eGFR, platelet count and LDH level remained stable following the switch to ravulizumab, and no new events of dialysis, kidney transplantation or thrombotic microangiopathy relapse were reported. “These results support the real-world effectiveness of ravulizumab in patients with aHUS who switched from eculizumab,” concluded the authors.3
Two posters by Yan Wang and colleagues at Alexion-AstraZeneca Rare Disease used the Adelphi aHUS Disease Specific Programme, a third-party database that integrates available real-world data, to evaluate clinical and patient-reported outcomes in patients initiating de novo ravulizumab or switching from eculizumab. Switch patients experienced an overall improvement in key clinical markers of aHUS (serum creatinine, lactate dehydrogenase, and platelets) compared to their prior treatment; ravulizumab was also effective at improving these parameters from baseline in previously untreated patients. (Figure 1) These clinical improvements translated into benefits for patient quality of life, with the physician-reported physical health and mental health of patients frequently being rated “good” to “excellent.”4
Figure 1. Clinical outcomes with ravulizumab in treatment-naïve patients and patients switching from eculizumab
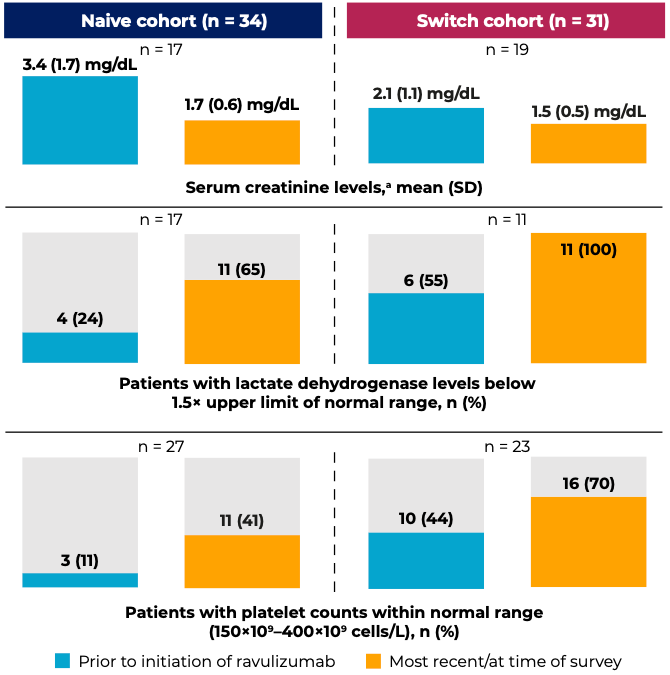
Data indicated as “most recent/at time of survey” were from the time the physicians extracted patient-level data.
aNormal serum creatinine levels are considered to be 0.7–1.3 mg/dL for men and 0.6–1.1 mg/dL for women.
Adapted from: Wang Y et al. Poster SA-PO920 at Kidney Week 2023.
The second poster drew on the same Adelphi dataset to evaluate patients’ and physicians’ satisfaction with ravulizumab treatment. Among physicians, overall satisfaction with ravulizumab was high across both switch and de novo ravulizumab patients; no physician was unsatisfied with ravulizumab treatment. Patient-reported satisfaction was also high, with 86% reporting they were more satisfied with ravulizumab than their prior eculizumab, and 14% rating the two options as equally satisfactory. 92% of physicians cited the lower number of infusions as a key motivation to switch; 52% mentioned a reduced burden on the patient and caregiver and 32% noted the improvement in efficacy. The authors concluded, “There was concordance in high satisfaction with ravulizumab between physicians and patients in the naive and switch cohorts; no dissatisfaction with ravulizumab was reported.”5
When asked about the most important considerations for Canadian clinicians using eculizumab or ravulizumab to manage aHUS, Dr Licht said that the appropriate duration of treatment, and how to manage treatment discontinuation and recurrence risk, is a “hot topic.” “We have an understanding of the clinical risk profile [in aHUS], and it leads to the guidance on how long to treat the middle-of-the-road cases. It will likely be only the exceptional cases that will be long-term treated,” he explained.
Moving toward a more personalized treatment approach
As in many other fields of medicine, in aHUS our evolving understanding of pathophysiology and biomarkers may soon be able to help us provide a “precision medicine” approach, by identifying patients at high risk or those likely to respond to treatment.
Several presentations highlighted the likely importance of deficiencies in complement factor H (CFH) as a risk factor for aHUS. CFH is a key regulator of the alternative complement pathway, with a major role in protecting the body’s own cells from complement-mediated damage. In the Global aHUS Registry study by Schaefer et al., among individuals with aHUS who switched to ravulizumab the most common pathogenic variant was a CFH mutation, present in about one-third of adults and one-quarter of children.3 Two additional case studies provided further support for CFH deficiency as a contributing factor to aHUS:
- A patient who developed TMA following a kidney transplant was found to have a CFH mutation, which complicated management of the TMA in the context of multiple other triggers. The patient eventually responded well to eculizumab and belatacept; the authors mentioned the CFH mutation as “a valuable guide for continued use of eculizumab”6
- A patient presented with post-partum TMA that was initially suspected to be thrombotic thrombocytopenic purpura; detection of low CFH levels led to the diagnosis of aHUS, and treatment with eculizumab was successful7
Asked to comment on the potential of personalized medicine in aHUS and how data from the Global aHUS Registry might support this approach, Dr Licht said, “The big picture will be impacted by [the Registry’s ongoing] in-depth effort to link outcomes more reliably to the genotype, including reported mutations and presence of antibodies. My prediction is that, for most of the questions that we have already spoken about, it will lead to a rewriting or kind of a reanalysis of the data, and we'll be able to say something about genotype-phenotype correlations. And I think that is quite important, since it gives us an opportunity to become more predictive, and [to look at] what the expected outcome is in patients [with a specific genotype].”
References
1. Boynton SA, et al. Poster FR-PO652 at Kidney Week 2023, November 2–5, 2023.
2. Coward R, et al. Translational Session: Complement as a target in CKD. Oral presentation at Kidney Week 2023, November 2–5, 2023.
3. Schaefer F, et al. Poster SA-PO922 at Kidney Week 2023, November 2–5, 2023.
4. Wang Y, et al. Poster SA-PO920 at Kidney Week 2023, November 2–5, 2023.
5. Wang Y, et al. Poster SA-PO921 at Kidney Week 2023, November 2–5, 2023.
6. Capistrano MC, et al. Poster FR-PO760 at Kidney Week 2023, November 2–5, 2023.
7. Ho T, et al. Poster TH-PO817 at Kidney Week 2023, November 2–5, 2023.