Reports
Evolving Landscape in the Treatment of Multiple Sclerosis
This report is based on medical evidence presented at sanctioned medical congress, from peer reviewed literature or opinion provided by a qualified healthcare practitioner. The consumption of the information contained within this report is intended for qualified Canadian healthcare practitioners only.
MEDICAL FRONTIERS - 5th Joint Triennial Congress of the European and Americas Committees for Treatment and Research in Multiple Sclerosis (ECTRIMS/ACTRIMS)
Amsterdam, The Netherlands / October 19-22, 2011
Amsterdam - The development of disease-modifying therapies in multiple sclerosis (MS) has been rapidly evolving over the past few years with approvals in the 1990s of the first-generation drugs, beta-interferons and glatiramer acetate, to now oral agents for the long-term treatment of MS. While oral agents appear efficacious and may provide better patient compliance, some safety concerns remain. At this year’s ECTRIMS/ACTRIMS, data were presented from pivotal trials of 5 oral agents in late-stage development for the treatment of relapsing-remitting MS. Among emerging oral agents, only the once-daily immunomodulator laquinimod may have the potential to access the CNS and limit demyelination and axonal damage through both direct and indirect mechanisms. In addition, new data presented showed how the role of the established first-line therapies is evolving within the increasingly complex MS therapeutic landscape.
At this year’s ECTRIMS/ACTRIMS, tributes were paid to one of the pioneers in investigational treatments for multiple sclerosis (MS), Dr. Kenneth P. Johnson, formerly Chairman of Neurology, University of Maryland School of Medicine, Baltimore, who passed away on September 3, 2011. Dr. Omar Khan, Wayne State University School of Medicine, Detroit, Michigan, US, recalled how Dr. Johnson’s work paved the way for the clinical trials that led to the approval of subcutaneous interferon beta-1b (IFNb-1b SC) and glatiramer acetate (GA) for the treatment of relapsing-remitting MS (RRMS). “The US pivotal study, which led to approval of GA worldwide, has continued in an open-label fashion… and it has translated into the longest prospective study in MS, currently entering its 20th year, showing sustained clinical benefit without compromising safety,” Dr. Khan declared.
Delaying Disease Onset: Established Results
GA and IFNb have been shown in 5 pivotal randomized, controlled clinical trials to be effective in delaying MS onset when initiated after a first clinical demyelinating event indicating clinically isolated syndrome (CIS). “This has already led to a paradigm shift towards earlier treatment,” observed Prof. Giancarlo Comi, Università Vita-Salute San Raffaele, Milan, Italy. He compared methodologies, baseline patient characteristics and primary outcomes in the trials of IFNb-1a (CHAMPS, ETOMS, REFLEX), IFNb-1b (BENEFIT) and GA (PreCISe). Despite differences in study design, end point definition and recruitment environment, all studies showed significant reductions in the risk of clinically definite MS (CDMS) with GA or IFNb. In the placebo groups, 38% to 45% of patients converted to CDMS compared with 20% to 34% of patients on active treatment. Significant reductions were also reported in the proportion of patients who attained McDonald criteria MS within 2 years of the first event.
According to the results of a meta-regression analysis of REFLEX, PRISMS and SPECTRIMS, 3 trials that compared IFNb-1a SC high-dose (44 μg t.i.w.) and low-dose (22 μg t.i.w. or 44 μg q.w.) with placebo, the benefit of disease-modifying therapy (DMT) is greater in patients with CIS than in patients with RRMS or secondary progressive MS (SPMS). Dr. Maria Pia Sormani, University of Genoa, Italy, reported that SC IFNb-1a significantly reduced the risk of relapse in patients with CIS by 47% with low dose (P=0.002) and 52% with high dose (P<0.001). Reductions were smaller, but still significant, in patients with RRMS. In patients with SPMS, reductions were even smaller and only significant with the higher dose. Analysis adjusted for dose showed a 28% (P=0.05) reduction of treatment effect passing from CIS to RRMS and from RRMS to SPMS.
Functional Benefits
Data demonstrating the beneficial effects of GA treatment beyond the established effect on clinical disease activity and safety profile were presented from the Coptimize and QualiCop studies by principal investigator, Prof. Tjalf Ziemssen, Universitätsklinikum Carl Gustav Carus, Dresden, Germany. Coptimize is an ongoing international, non-interventional, longitudinal study of RRMS patients switching to GA from any MS drug within 3 to 6 months of screening.
In Coptimize, preliminary analysis of 618 patients who switched to GA showed that most patients reported better efficacy and safety (P<0.0001). Of patients reaching the 12-month follow-up after switching to GA, the annualized relapse rate (ARR) was reduced by almost two-thirds (P<0.0001) and no progression of Expanded Disability Status Scale (EDSS) scores. “The Coptimize study can help physicians evaluate their clinical data in comparison with emerging international reference benchmarking,” Prof. Ziemssen suggested.
Significant improvements in cognitive function and depressive symptoms were observed in patients taking GA in QualiCop, an observational, open-label non-interventional study conducted in 734 patients with RRMS. All patients underwent a series of 11 examinations over 2 years using various assessments and questionnaires. “Compared to the data before study entry, our findings indicated that treatment with GA resulted in a stabilization of most quality-of-life (QoL) parameters, with slightly improved overall MS Functional Composite scores after 24 months of treatment with a robust improvement in cognition shown in the Paced Auditory Serial Addition Test (PASAT) and the Multiple Sclerosis Inventory Cognition (MUSIC) test,” Prof. Ziemssen reported. Fatigue measurement was stable on an overall low level by the fatigue scale for motor and cognitive functions and MUSIC test. Depression as measured by a general depression scale (long version) showed a significant improvement over 24 months. “QoL, cognition and fatigue during immunomodulatory therapy with GA may be equally important to patients as are reductions in relapse rate and disease progression,” Prof. Ziemssen suggested. He believes that these benefits are really important in real life for MS patients and can improve compliance and adherence to therapy and consequently increase treatment efficacy.
The sustained clinical benefit of GA and impact on QoL also continues to be confirmed in daily clinical practice. Dr. Pavel Stourac, Masaryk University, Brno, Czech Republic, reported a multicentre observational study involving patients with RRMS treated with GA 20 mg q.d. between 2006 and 2010 and followed for 13 months. Among 746 patients, 167 had been switched to GA from IFN because of intolerable side effects or lack of efficacy. After 1 year of treatment with GA, ARR decreased to 0.46 from 1.8 and average EDSS score was reduced from an average of 2.63 to 2.54. Patients’ answers to QoL questionnaires showed significant improvements in family relations, health worries, tiredness and conservation of energy (P<0.05). Fatigue impact scale questionnaires revealed significant improvements in flexibility, social isolation, working ability, motivation, concentration and need for frequent rest (P<0.01 to 0.04).
New Targets for Future Treatments
Currently approved immunomodulatory or immunosuppressive therapies for MS mainly target different components of the peripheral immune system, especially the T-cell-mediated inflammatory pathway that is primarily responsible for the development of focal lesions and relapses. Other therapeutic targets within the central nervous system (CNS) should be considered to broaden the capacity to alleviate the disease, remarked Prof. Wolfgang Brück, University Medical Centre Göttingen, Germany. Indeed, key neurodegenerative processes appear to play a significant role in disease progression, largely independent of the inflammatory immune-response originating from the periphery. Those processes are responsible for the chronic underlying diffuse neurodegeneration (neurodegeneration occuring through all the CNS parenchyma) being a major cause for long-term, permanent disability.
These targets may include CNS-resident inflammation, inhibition of myelin and axonal damage, or even the repair of MS lesions. The novel once-daily oral immunomodulating agent laquinimod passively crosses the blood-brain barrier and has direct effects on the CNS inflammatory/degenerative pathways involved in the pathogenesis of MS. It has been shown to improve and moderate experimental autoimmune encephalomyelitis (EAE), the principle animal model of MS. Preventive and therapeutic treatment with laquinimod significantly reduced spinal cord demyelination in this model. It also significantly reduced axonal damage in the spinal cord of EAE mice. New data reported in this model showed that after oral administration, laquinimod was present in CNS tissues of EAE and healthy mice up to 12 h post-dose and that brain:blood ratios were higher in the EAE mice.
Prof. Brück also presented the cuprizone model of demyelination which causes a direct CNS pathology that is not a result of a breach in the blood-brain barrier (unlike the demyelination in MS and EAE) and does not involve T-cells from the periphery. In this model of demyelination, preventive treatment with the oral agent has been shown to be effective in reducing demyelination, inflammation, axonal damage and oligodendroglial pathology. Dr. Christiane Wegner, Georg-August Universität, Göttingen, presented data from a study in which 10-week-old male wild-type C57BL/6J mice and mice deficient in recombination activating gene 1 (RAG-1) challenged with cuprizone feeding and treated daily with laquinimod 25 mg/kg for 6 weeks displayed significantly less demyelination than untreated controls. The treated mice had significantly lower callosal densities of macrophages and T-cells and they displayed significantly less axonal damage in the callosal white matter. “Our results indicate that laquinimod appears to have beneficial effects on myelin and oligodendrocytes,” Dr. Wegner noted. “These effects are independent of T-cells as indicated by the findings in RAG-1-deficient mice.”
Prof. Brück pointed out that activation of astrocytes and microglial cells is prevented by laquinimod in this model. Results from the cuprizone model suggest that laquinimod’s ability to inhibit astrocyte activation and prevent demyelination and axonal damage results from its direct effect within the CNS and is independent of its action on peripheral immune cells. In addition, this model may suggest that laquinimod has an effect on the diffuse neurodegeneration occurring in the CNS, and not only on the focal lesions/tissue damage caused by activated T-cells infiltrating from the periphery.
It has been suggested that it inhibits pro-inflammatory cytokine secretion by these cells by interference with the NFkB pathway within the CNS, resulting in a decrease in toxic intermediate molecules that lead to death and damage to oligodendrocytes and neurons. “This mode of action supports the clinical results demonstrating preservation of brain tissue and delay in disability progression with laquinimod,” Prof. Brück reported.
ALLEGRO and BRAVO
Key data that supported this mechanism of action were presented from 2 recently completed phase III clinical trials. The main results of the 2-year ALLEGRO trial, which randomized 1106 RRMS patients to placebo or laquinimod 0.6 mg q.d., showed that actively-treated patients had a 23% reduction in ARR vs. placebo (P=0.0024) (Comi G. Neurology 2011;76[9 suppl 4]:7PP.001). Also, there was a reduction in the risk of progression of EDSS of 36% (P=0.0122) and 48% (P=0.0023) sustained for 3 and 6 months, respectively (Figure 1).
Figure 1.
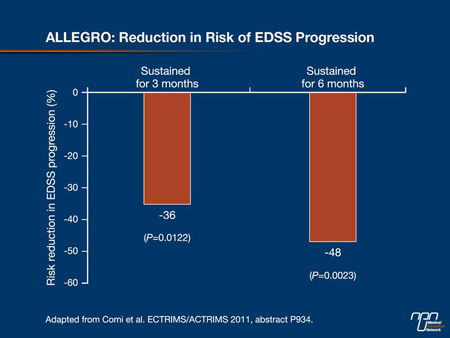
This observation was reinforced by a 35% reduction in risk of confirmed progression (sustained for 3 months) and the persistence of EDSS change at last observed value (P=0.036). The proportion of patients with confirmed EDSS progression after 24 months was 9.8% for laquinimod vs. 14% for placebo (P=0.038). New exploratory analyses presented by Prof. Comi also showed that the ARR requiring intravenous steroids or hospitalization was 27% and 38% lower in the laquinimod patients compared to those on placebo (both P<0.0001). Active treatment confirmed its effects with a 33% reduction (P<0.0001) in MRI-measured brain atrophy. ALLEGRO also showed a significant difference in magnetization transfer ratio indicating a progressive increase in structural damage of global brain tissue in placebo patients vs. tissue stabilization in laquinimod patients.
The ALLEGRO trial also revealed a significant effect of laquinimod on patient-reported fatigue. Dr. Douglas R. Jeffery, Cornerstone Health Care, Advance, North Carolina, reported that fatigue as measured by the Modified Fatigue Impact Scale worsened in the placebo patients (34.4) compared with the laquinimod group (31.9) with a treatment effect of -2.53 (P=0.004) (Figure 2). Functional status, measured by the short-form (SF)-36 general health survey, also showed a significant treatment effect difference vs. placebo on the mental component summary and the physical component summary remained stable for laquinimod whereas it declined for the placebo group. “The finding that fatigue and functional status are positively influenced by laquinimod, together with the positive effects on clinical and MRI measures of disease burden, indicate that laquinimod has an impact on the neurodegenerative processes of MS,” Dr. Jeffery concluded.
Figure 2.
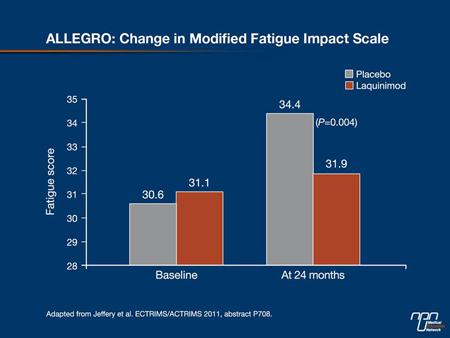
In the 2 patient groups, adverse events (AEs) were similar with only minor abdominal pain and back pain reported more frequently with laquinimod. More patients on active therapy had elevated liver enzymes, but these were mild and transient and normalized. There were no serious AEs such as cancer or serious infections. “The drug was very safe and very well tolerated.” Prof. Comi remarked. In a substudy in 100 patients, no changes were found during the trial in peripheral blood mononuclear cell composition or in proliferative response to mitogen or recall antigen in patients receiving laquinimod, showing that they retained response capacity to immunological stimuli.
With a similar design to ALLEGRO, the BRAVO study had an additional reference arm of IFNb-1a (IM). Results were consistent with ALLEGRO, showing “a robust effect on parameters of neurodegeneration (progression of disability and brain volume loss)” and “a good safety and tolerability profile,” stated Dr. Timothy L. Vollmer, University of Colorado Denver School of Medicine, Aurora.
In BRAVO, 1331 patients with RRMS were randomly assigned to receive placebo, laquinimod 0.6 mg q.d. or IFNb-1a IM 30 µg/week. The primary end point, ARR— adjusted for baseline EDSS, number of relapses in the 2 years pre-study and country—was reduced by a non-statistical difference of 18% vs. placebo. However, after further adjustment for T2 lesion volume and percentage of patients with gadolinium-enhanced (GdE) T1 lesions, predictors of on-study relapse rate that were not balanced between groups, ARR reduction was 21% (P=0.026) for laquinimod and 29% for IFNb-1a IM (P=0.002).
Compared with placebo, the oral agent was associated with a significant reduction in the risk of disability progression as measured by the EDSS (HR=0.665; 95% CI, 0.447-0.989, P=0.044), whereas reductions with IFNb-1a were not statistically significant. On laquinimod, MRI-measured brain volume loss was reduced by 27.5% (P<0.0001), with no effect for patients on IFNb-1a (Table 1). “Consistent with the ALLEGRO findings, these effects appear to be independent from its anti-inflammatory properties.” Dr. Vollmer noted.
Table 1.
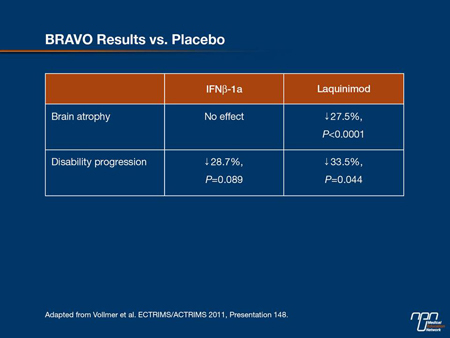
There was 1 death in each of the laquinimod and IFNb-1a groups, neither of which was believed to be treatment-related. Two cases of malignant neoplasms were reported in each of the active treatment groups. As in ALLEGRO, a higher incidence of back pain was reported compared with the placebo and IFNb-1a groups; these were generally mild, tended to resolve shortly after treatment and did not lead to discontinuation in most patients. Also, the noted elevations of liver enzymes were mild with no evidence of liver injury. “These data suggest that this drug may have an important role to play in the future treatment of MS patients,” Dr. Vollmer told delegates.
Commenting on the BRAVO study during the highlights session of the meeting, Prof. Ralf Gold, Ruhr-University, Bochum, Germany, stated, “Laquinimod seems to be absolutely safe. The disability is efficiently stopped by laquinimod and, importantly, brain atrophy is stopped. I am quite confident that we will see further studies on laquinimod in the future and at higher dosages.” An extension study of BRAVO to evaluate its long-term effects and safety is ongoing.
Expanding Armamentarium of Oral DMTs
BG-12, an oral formulation of dimethyl fumarate, was associated with significant reductions in relapse rates and progression of disability in the international phase III DEFINE study, according to lead investigator Prof. Gold. The study randomized 1234 patients in double-blind fashion to placebo or treatment with BG-12 240 mg b.i.d. or t.i.d. After 96 weeks, both doses reduced the cumulative risk of relapse, the primary end point of the study, by 49% and 50%, respectively (both P<0.0001 vs. placebo (Figure 3). “The placebo group diverged from the treatment groups around month 3, so it may take some time to get full activity with BG-12,” Prof. Gold noted.
Significant effects were seen on all secondary efficacy end points, including time to confirmed 12-week disability progression and ARR at 2 years. Both active doses significantly reduced mean numbers of GdE lesions and new/newly enlarged T2 lesions. The overall incidence of AEs and serious AEs was similar among the placebo group and both active treatment groups.
Figure 3.
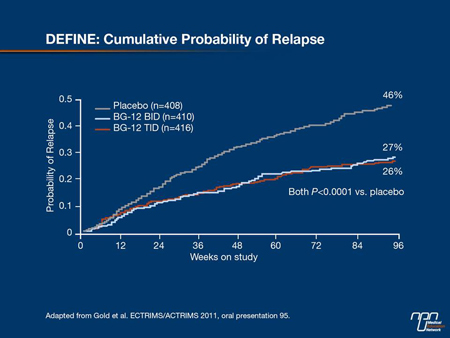
Consistent with phase II studies, patients reported flushing more frequently with BG-12 at 35% of patients overall vs. 5% in the placebo group. Also, withdrawal from the study because of AEs was higher vs. placebo (16% vs. 13%). “These results suggest that BG-12 may provide a therapeutic option for patients with relapsing MS with robust efficacy, a good tolerability and safety profile for this oral drug,” Prof. Gold added. Similar results were reported from CONFIRM, a second phase III trial of BG-12, which included a GA reference comparator patient group.
Long-term beneficial effects of teriflunomide were reported from an interim analysis of data from the TEMSO extension study. In the main TEMSO trial, teriflunomide 7 or 14 mg q.d. for 108 weeks reduced AAR by 31% (P<0.001 vs. placebo), reduced 12-week-confirmed disability progression and improved MRI markers of disease activity (O’Connor et al. N Engl J Med 2011;365:1293-303). The long-term, double-blind extension study involved 742 patients who completed the core study, all continuing on 7 mg or 14 mg. Lead investigator Dr. Paul O’Connor, University of Toronto, Ontario, Canada, reported that 5 years after randomization in the main trial, the risk of 12-week sustained disability progression was numerically lower in patients initially treated with the oral agent than with patients initially on placebo. ARR remained low in all treatment groups. Changes from baseline in total MRI lesion volume were numerically lower in the patients originally randomized to active therapy.
The first orally administered agent to be approved worldwide for the treatment of RRMS is fingolimod. Dr. Eva Havrdová, Charles University, Prague, Czech Republic, reported post-hoc analyses of data from FREEDOMS and TRANSFORMS, respectively 1-year and 2-year phase III studies that compared it with IFNb-1a (IM) in patients with RRMS. The analyses confirmed that fingolimod 0.5 mg improved clinical outcomes compared with placebo or IFNb-1a IM in all subgroups of patients with active disease, including those previously treated with IFNb-1a and treatment-naive patients with rapidly evolving severe MS.
New Therapeutic Monoclonal Antibodies
A 5-year observational study to evaluate the long-term safety and treatment impact of the a4-integrin antagonist natalizumab in patients with RRMS is ongoing in Europe, Australia and Canada. According to an analysis of 3484 patients (as of June 1, 2011) reported by Prof. Heinz Wiendl, University of Münster, Germany, ARR was significantly reduced and remained low after 3 years of treatment and EDSS scores remained stable at 3.4. Mean ARRs were lowest in patients who were treatment-naive at baseline and highest in patients who were previously treated with immunosuppressant therapy. The cumulative probability of sustained EDSS improvement was 19% compared with 11% for sustained EDSS progression. “These findings suggest a potential benefit of treatment with natalizumab early in the course of the disease,” Prof. Wiendl told delegates.
Although phase III trials showed beneficial effects of natalizumab, they also revealed an increased rate of brain volume loss compared to placebo during the first year of therapy and a reduced rate of volume loss in the second year. This has been attributed to “pseudoatrophy,” i.e. shrinkage of brain after cessation of the inflammatory process, noted Dr. María José Magraner, Hospital Universitari i Politècnic La Fe, Valencia, Spain. From an 18-month investigation of 18 natalizumab-treated RRMS patients, Dr. Magraner reported reductions of 67% in ARR and 87.5% in cumulative GdE lesions accompanied by a decrease in brain volume of 2.5%. This occurred mainly in the peripheral grey matter, with 75% of the atrophy occurring during the first 6 months of therapy. Brain atrophy was related to the number of previous relapses and with inflammatory activity during treatment. “This could indicate pseudoatrophy due to inflammation-related changes or real axonal loss,” Dr. Magraner speculated.
Daclizumab, a humanized monoclonal antibody (MAb) specific for the a-subunit (CD25) of the high-affinity interleukin-2 receptor, showed a clinically meaningful effect on disease activity over 52 weeks, according to Prof. Gavin Giovannoni, Queen Mary University of London, UK. In the phase IIb SELECT study, 600 patients with RRMS and ≥1 MS relapse during the previous year or 1 new GdE lesion during the previous 6 weeks were randomly assigned to receive daclizumab high-yield process (HYP) 300 mg or 150 mg or placebo administered SC q4 weeks. Compared with placebo, both HYP doses were associated with significant decreases (50% to 54%) in ARR, and approximately 80% of MAb patients were relapse-free compared with 64% the placebo group. “A surprising result, considering that it was only a 52-week study, was the 43% to 67% reduction in confirmed disability progression,” Prof. Giovannoni remarked. The rate of AEs was similar among the treatment groups, although 1 death occurred in the trial, possibly related to daclizumab HYP treatment. “These results warrant further study of daclizumab HYP in large trials,” Prof. Giovannoni concluded. A phase III trial, DECIDE, has already begun, comparing daclizumab 150 mg with IFNb-1a in approximately 1500 RRMS patients.
In an extension of the phase II CAMMS223 study, RMMS patients treated with alemtuzumab, a humanized MAb directed against the cell-surface protein CD52, exhibited less clinical disease activity than patients treated with IFNb-1a 4 years after receiving the last treatment cycle. Prof. Alexey Boyko, Moscow Multiple Sclerosis Center and Russian State Medical University, reported that the extension phase involved 116 patients randomly assigned to receive alemtuzumab 12 or 24 mg/day (IV) during 2 annual cycles and 47 patients to IFNb-1a (SC) 44 µg t.i.w. in the main study. At year 5 post-randomization to treatment, MAb patients had a 69% lower risk for sustained accumulation of disability (SAD) relative to IFN patients and ARR was 0.12 for alemtuzumab and 0.35 for IFNb-1a. Estimates for patients SAD-free, relapse-free and clinically disease-free were all significantly greater for alemtuzumab vs. IFNb-1a (P<0.0001). Mean EDSS disability scores improved from baseline by 0.35 points with alemtuzumab but worsened by 0.46 point with IFNb-1a. “These results suggest durability of response to alemtuzumab treatment with significant effect still evident 4 years after the last dose,” Prof. Boyko said.
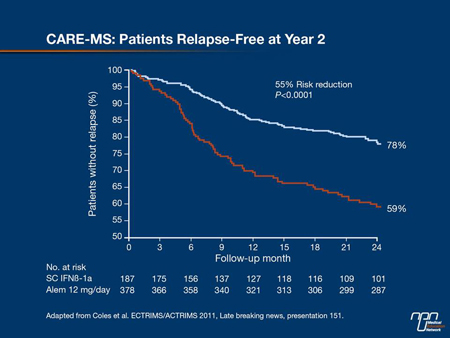
The efficacy and safety of a 5- and 3-day cycle of alemtuzumab 12 mg/day (IV) annually or 3 injections of IFNb-1a 44 μg q.w. SC over 2 years have been compared in 563 treatment-naive patients with recent disease activity in a phase III study, CARE-MS I. Lead investigator Dr. Alasdair J. Coles, University of Cambridge, Addenbrooke’s Hospital, UK, reported that alemtuzumab met one of the 2 co-primary end points of relapse rate reduction of 55% compared with IFNb-1a SC (P<0.0001) (Figure 4). But the differences between time to 6-month SAD and other EDSS-based end points were not significant between alemtuzumab and IFNb-1a SC. These results confirmed the beneficial effects seen in previous studies with alemtuzumab on relapse rates, but not on disability, which may have been related to the low number of patients with high disability in the IFNb-1a SC group, Dr. Coles suggested. No new safety signals emerged in this study. Dr. Coles noted that CARE-MS II is studying the same dosing arms in addition to a third arm, alemtuzumab 24 mg/day, in treatment-experienced RRMS patients. This trial is expected to be completed late 2011.
Figure 4.
Summary
While there is still no cure for MS, proven first-line disease-modifying agents have established their role in effectively delaying disease onset, reducing relapses and disease progression. Equally important is the long-term experience accumulated with those treatments in both randomized clinical trials and real-life studies, and the proven improvement of QoL, cognition and fatigue which can also improve compliance and adherence to therapy and consequently increase treatment efficacy beyond clinical disease activity. Even more encouraging is the number of candidate drugs in clinical development trials, with some of them, like laquinimod, having potentially interesting activity within the CNS on neurodegenerative processes that collectively will improve MS patient outcomes.