Reports
Invasive Fungal Infections in Severely Immunocompromised Patients: Strategies to Improve Overall Survival
This report is based on medical evidence presented at sanctioned medical congress, from peer reviewed literature or opinion provided by a qualified healthcare practitioner. The consumption of the information contained within this report is intended for qualified Canadian healthcare practitioners only.
PRIORITY PRESS - CACMID-AMMI Canada 2012 Annual Conference
Vancouver, British Columbia / May 3-5, 2012
Vancouver - Invasive fungal infections (IFIs) affect mainly patients with hematological malignancies undergoing hematopoietic stem cell transplantation. Invasive aspergillosis (IA) accounts for the majority of these infections and it can be detected early using either the galactomannan or the beta-D-glucan assay. Early, diagnosis-driven introduction of antifungal therapy allows physicians to treat patients pre-emptively when the infection is still local and survival odds are greatest. Until recently, outcomes using combination antifungal agents for the treatment of proven or probable IA have not been convincingly better than single agents alone. However, the first randomized, double-blind study of combination antifungal therapy vs. a single-agent antifungal in probable/proven IA has shown a trend in improved survival, but did not reach statistical significance. These findings suggest there may be more effective strategies for the treatment of this important infection in severely immunocompromised patients.
Chief Medical Editor: Dr. Léna Coïc, Montréal, Quebec
The occurence of invasive fungal infections (IFIs) are common in immunosuppressed patients and are predominantly caused by the Aspergillus species. “It’s thus no surprise that the 2 populations most at risk for invasive mold infections (IMIs) are transplant patients and patients with hematological malignancies,” Dr. Michel Laverdière, Université de Montréal, Quebec, told delegates here. Incidence studies suggest that some 3.4% of hematopoietic stem cell transplant (HSCT) recipients develop an IFI over the course of a year; approximately two-thirds of these are mold infections. Similar numbers of solid organ transplant recipients also develop an IFI over time but approximately two-thirds of them are yeast infections. However, lung transplant recipients are far more prone to IMIs than yeast infections, as Dr. Laverdière noted.
Current diagnostic techniques to identify early IMIs are less than ideal. The most accurate diagnostic techniques for detecting an IFI are often too risky to be carried out in severely immunocompromised patients and establishing an etiology based on imaging studies is challenging, as Dr. Laverdière indicated.
The assay detecting Aspergillus galactomannan (GM), an antigen present in the mold’s cell wall, is highly specific for aspergillosis but the less specific beta-D-glucan test can also be very helpful; in his opinion, both tests are “quite comparable” in terms of their predictive capacity, at least in neutropenic patients. The predictive value of GM in bronchoalveolar lavage fluid (BAL) is also good in HSCT and in lung transplant recipients. “Outside of these 2 settings, the predictive value of the GM in BAL is not as good,” Dr. Laverdière stated.
Early Diagnosis, Therapy Critical
The importance of establishing an early diagnosis of invasive aspergillosis (IA) using more recent diagnostic techniques cannot be overstated. As discussed by Prof. Ben de Pauw, Emeritus Professor of Medicine, University Medical Center St. Radboud, Nijmegen, The Netherlands, mortality rates from IFIs almost approached 97% when physicians used traditional diagnosis techniques. Now, with the newer techniques including the GM and beta-D-glucan assays, mortality from IFIs may be as low as 22%, provided treatment is initiated early. As Prof. de Pauw explained to delegates, patients are already colonized so there is a risk of IA once they become neutropenic. And when they have febrile neutropenia, we give them antibiotics but, in fact, they could have an IA and require antifungal therapy, even if the diagnosis is not obvious.
Aspergillus is mainly an airborne infection and spores will enter the body through the airways and descend into the bronchi where the first lesions usually develop. Infection at this stage is local; it is only when the organism starts to invade not only the lungs but elsewhere that it causes invasive disease. In studies in which antifungal prophylaxis has been introduced before there was any sign of invasive infection, no advantage for prophylaxis was seen relative to placebo in either the incidence of IA or overall mortality, according to a meta-analysis of 38 clinical trials of prophylactic antifungal therapy (Bow et al. Cancer 2002;94:3230-46).
Prof. de Pauw stated that it has become standard of care to treat patients empirically when a fever does not subside after 3 to 4 days of broad-spectrum antibiotics. In point of fact, “under these circumstances, a clinically overt IFI is very rare,” even in the absence of empirical antifungal therapy, he stressed. The risk of patients developing an IFI is virtually nil among those who are not hemodynamically unstable, who do not have evidence of a localized infection on diagnostic testing and who are not otherwise considered high risk based on the presence of known risk factors.
Consequently, Prof. de Pauw argued for a far more restrained approach to the empiric treatment of IFIs to one that is diagnosis-driven, i.e. based on the identification of a fungal infection in the lungs, coupled with clinical consideration of known risk factors for IFIs, most notably acute graft-versus-host-disease and the presence of any type of pulmonary abnormality. Such an approach would allow physicians to treat an early infection “pre-emptively” and offer patients the greatest opportunity for improved survival with early initiation of antifungal therapy.
According to Prof. de Pauw, “If you have extensive experience with these infections and the diagnostic facilities available to identify IFIs early, the pre-emptive approach is the way to go.”
Combination Therapy for Proven/Probable IA
As an oncologist, Dr. Eric Bow, Professor of Medicine and Medical Microbiology, University of Manitoba, Winnipeg, identified a number of potential advantages to using a combination of antifungal agents for treating patients with proven or probable IA. These advantages include:
- the potential to enhance activity through synergy between 2 drug classes;
- a wider spectrum of antifungal activity;
- the prevention of resistance.
In a seminal study, initial therapy with voriconazole in patients with IA led to better responses and fewer severe side effects than the standard approach using amphotericin B (Herbrecht et al. N Engl J Med 2002;347(6):408-15). In the same study, significantly improved survival was also demonstrated in favour of the triazole antifungal. Based on this study and ongoing clinical experience, voriconazole is recommended for the primary treatment of IA in most patients (A-I) (IDSA Guidelines for Aspergillosis (CID 2008:46).
Until recently, studies exploring the potentially greater efficacy of a combination antifungal strategy vs. monotherapy for proven or probable IA have not produced convincing results, as Dr. Bow demonstrated. For this reason, investigators undertook the first prospective, randomized, double-blind trial of combination therapy vs. single-agent therapy for proven or probable IA in a cohort of HSCT recipients. Reported for the first time at the 2012 European Congress on Clinical Microbiology and Infectious Diseases (Poster LB 2812), patients in the study were randomized to voriconazole with or without anidulafungin, an echinocandin. Combination therapy was given for 2 to 4 weeks, after which patients could be switched to the triazole alone to receive a total of 6 weeks of antifungal therapy. “The primary end point of the study was all-cause mortality at 6 weeks,” Dr. Bow reported. A total of 142 patients with proven or probable IA were randomized to voriconazole alone and 135 patients to the combination arm.
Probable IA was identified in 98% of the cohort, and 80% of the diagnoses were driven by GM positivity, he added. By
week 6, mortality was lower although not significantly so (P<0.09) in the combination arm at 19% vs. 28% for the monotherapy arm in the overall intent-to-treat cohort. This represented a relative risk reduction of approximately 30% with a number-needed-to-treat (NNT) to prevent 1 death of 12.
Figure 1.
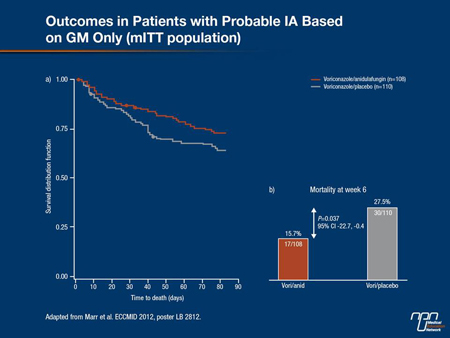
Similarly, the 6-week all-cause mortality difference among patients with GM-defined IA was statistically significant (P=0.037) at 15.7% in the voriconazole/anidulafugin arm compared to 27.5% for the triazole-alone arm (Figure 1). As Dr. Bow pointed out, “this represented a relative risk reduction of 42% with an NNT of 7. With no major signals that safety outcomes for those in the combination arm were different from those in the single-agent arm, “this is the first randomized control trial to demonstrate a survival effect [from a combination strategy], with an absolute risk reduction in mortality of about 12% and an odds ratio reduction in mortality of about 50%,” Dr. Bow told delegates. “So this is quite different from many of the other trials we have seen.”