Reports
Progress in the Treatment of Drug-resistant Nosocomial Pneumonia
This report is based on medical evidence presented at sanctioned medical congress, from peer reviewed literature or opinion provided by a qualified healthcare practitioner. The consumption of the information contained within this report is intended for qualified Canadian healthcare practitioners only.
PHYSICIAN PERSPECTIVE - Viewpoint based on presentations from the 49th Annual Meeting of the Infectious Diseases Society of America (IDSA)
Boston, Massachusetts / October 20-23, 2011
Reviewed and edited by:
Ethan Rubinstein, MD, LLB
H.E. Sellers Research Chair in Infectious Diseases
Head, Section of Infectious Diseases
Department of Internal Medicine
University of Manitoba
Winnipeg, Manitoba
Introduction
Over the past two decades, methicillin-resistant Staphylococcus aureus (MRSA) has evolved from an infrequent source of pneumonia to a leading cause of severe pneumonia, particularly in hospitalized patients. Recent studies suggest that MRSA accounts for up to 40% of all hospital-acquired and ventilator-associated pneumonia (HAP, VAP). The severe forms of pneumonia associated with MRSA confer a high risk of mortality, exceeding 30% in many studies. Because of the ominous nature of MRSA, physicians should have a low threshold for suspecting a resistant organism in patients with HAP or VAP.
Studies have shown that early, appropriate and adequate treatment of methicillin-resistant Staphylococcus aureus (MRSA) pneumonia can reduce the mortality risk by as much as 50% and that delaying or using inappropriate therapy increases the risk.
Until fairly recently, vancomycin represented the only antibiotic available for MRSA pneumonia. Clinical experience with this antibiotic has been characterized by low cure rates and a high risk of mortality, principally due to vancomycin’s poor penetration in lung tissue. Whether higher serum concentrations would improve results remains unclear. Additionally, high doses of vancomycin can increase the risk of adverse renal effects.
Updated clinical guidelines from the Infectious Diseases Society of America (IDSA) specify vancomycin, linezolid and clindamycin as therapeutic options for hospital-acquired pneumonia (HAP) and ventilator-associated pneumonia (VAP) associated with MRSA (Liu et al. Clin Infect Dis 2011;52:1-38). The clindamycin recommendation applies only to children, as data on use in adults are limited.
The oxazolidinone linezolid achieves better penetration of lung tissues and lung epithelial lining fluid than vancomycin. Results of randomized trials have suggested that it is not inferior to vancomycin.
Recent studies have shed more light on the relative efficacy of vancomycin and linezolid in the treatment of HAP and VAP caused by MRSA. Data reported at the IDSA meeting provided new information about existing agents for MRSA and potential new options to anticipate for the future. Collectively, the new information offers some reason for optimism about improving outcomes in MRSA pneumonia but also underscores the need for ongoing research to learn more about the disease and expand the therapeutic options.
Trial Outcomes in MRSA Pneumonia
Two large, prospective, randomized clinical trials reviewed both antimicrobials in patients with staphylococcal VAP and HAP (Rubinstein et al. Clin Infect Dis 2001;32:402-12, Wunderink et al. Clin Ther 2003;25:980-92). Both trials were statistically powered to evaluate the non-inferiority of linezolid to vancomycin and similar clinical cure rates and pathogen eradication rates between treatment groups were demonstrated. However, while the numbers of patients with MRSA in both trials were small, an unplanned post-hoc analysis of both trials showed a higher eradication rate with linezolid in patients with MRSA.
A more recent randomized clinical trial of vancomycin and linezolid was conducted and enrolment was limited to patients with MRSA nosocomial pneumonia (Wunderink et al. Clin Infect Dis 2012;54:621-9). The trial included 1184 patients with HAP or health care-associated pneumonia (HCAP). This term refers to patients in nursing homes and non-hospital health care facilities, as well as patients undergoing outpatient procedures or treatment, patients recently discharged from hospitals and certain immunosuppressed patients (Anand N, Kollef MH. Sem Respir Crit Care Med 2009;30:3-9).
Patients were randomized to fixed-dose linezolid (600 mg q12 h) or dose-optimized vancomycin (15 mg/kg q12 h). The primary end point was clinical cure (resolution of signs and symptoms of pneumonia; improvement or lack of progression in chest imaging; and no requirement for additional antibacterial treatment) at the end of the study in evaluable patients treated according to protocol. Secondary end points included microbiologic outcome at end of study (EOS) and end of treatment (EOT), clinical outcome at EOT in the modified intent-to-treat (mITT) and per-protocol populations, and clinical outcome at EOS in the mITT population. Other secondary end points were patient survival and incidence of adverse events (AEs). The mITT analysis included 448 randomized patients and the per-protocol analysis included 348 patients.
In the per-protocol population, the clinical success rates were 57.6% with linezolid and 46.6% with vancomycin (95% CI, 0.5-21.6; P=0.042). Additionally, the linezolid group had a higher cure rate at end of treatment (83.3% vs. 69.9%) (Figure 1). Higher cure rates with linezolid at EOT and EOS were also shown in the mITT population.
Figure 1.
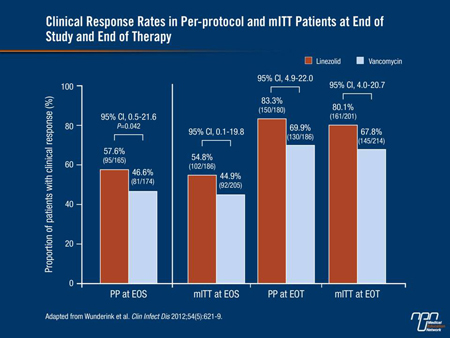
Overall, the incidence and type of AEs were similar between groups. Drug-related serious AEs occurred in 7 patients (8 events) treated with linezolid and 13 (18 events) in vancomycin-treated patients. Renal failure occurred in 3.7% and in 7.3% of patients, respectively, and nephrotoxicity (defined as a 0.5 mg/mL increase in normal baseline serum creatinine or a 50% increase if abnormal) occurred in 8.4% and 18.2%, respectively.
Renal toxicity was similar between treatment groups for patients with a baseline glomerular filtration rate (GFR) <50 mL/min but was higher with vancomycin in patients whose GFR exceeded 50 mL/min (18.8% vs. 5.6%). Renal toxicity in the vancomycin arm was greater if the day 3 trough level was ≥20 µg/mL (37%) compared with 15-20 µg/mL (22%) or <15 µg/mL (18%). The 60-day all-cause mortality was similar in the 2 groups.
In an accompanying editorial, Dr. Antoni Torres, University of Barcelona, Spain, described the results as a step forward in the battle against HAP and VAP associated with MRSA (Clin Infect Dis 2012;54:630-2). The trial was noteworthy for being the first randomized study to focus exclusively on patients with MRSA pneumonia.
While IDSA guidelines currently rate linezolid and vancomycin as similar, Dr. Torres called for reconsideration of linezolid’s role in MRSA pneumonia, given the cure rates achieved and the more favourable renal safety profile. Yet although it appears to represent an advance in the treatment of MRSA pneumonia, the percentages of clinical cure are still far from optimal.
Insights from IDSA
The IDSA annual meeting provides the forum for ongoing research in all aspects of infectious diseases, including MRSA pneumonia. A comprehensive review of relevant studies is not possible within the context of a brief publication. Nonetheless, the following presentations reflect laboratory and clinical work aimed at improving treatment for pneumonia and other infectious conditions caused by MRSA.
Organisms that have intermediate susceptibility or frank resistance to vancomycin pose a particularly difficult challenge. A need exists for more data about the activity of currently available antimicrobial agents that might represent viable therapeutic options when vancomycin achieves only a partial response or no response at all.
Saravolatz and colleagues (abstract 256) examined the activity of 13 different antibiotics against S. aureus strains that exhibited intermediate susceptibility to vancomycin (VISA) or resistance (VRSA). The drugs’ minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) were determined for 33 specimens with intermediate susceptibility and 13 that were resistant to vancomycin.
Four agents demonstrated >90% activity against the VISA strains: telavancin (100%), linezolid (100%), tigecycline (97%) and minocycline (94%). Analysis of the VRSA specimens showed that ceftaroline, daptomycin, linezolid, minocycline and trimethoprim/sulfamethoxazole were active against all of the strains. Tigecycline and rifampin had 92% activity. The remaining 6 drugs—ceftriaxone, telavancin, vancomycin, clindamycin, gemifloxacin and moxifloxacin—were not active against any of the resistant strains of S. aureus.
The investigators concluded that clinical experience with these agents is needed to determine which ones, if any, represent options for VISA and VRSA.
Targeting Empiric Therapy
The IDSA and American Thoracic Society (ATS) have published criteria for diagnosis of HAP, and the recommendations have demonstrated an acceptable balance of sensitivity and specificity for identifying patients who should be treated empirically (Am J Respir Crit Care Med 2005;171:388-416).
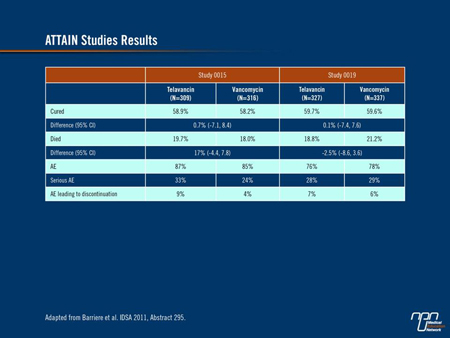
Barriere and colleagues retrospectively applied the ATS/IDSA criteria to patient populations of 2 identically designed phase III clinical trials of telavancin and vancomycin for adults with HAP caused by S. aureus and other gram-positive organisms (abstract 295). Of the 1503 patients included in the 2 trials, 1289 met the ATS/IDSA criteria. Investigators compared the 2 treatment arms for cure rates, on-study mortality and AEs. The analysis showed similar results for all of the outcomes assessed, supporting the trials’ original conclusion that telavancin is non-inferior to vancomycin (Table 1).
Table 1.
Salvage for Persistent MRSA Bacteremia
Park et al. retrospectively compared outcomes with linezolid-based and glycopeptide-based salvage therapy for patients with persistent MRSA bacteremia despite initial treatment with a glycopeptide antibiotic (abstract 570). Of 377 patients with MRSA bacteremia, 90 (24%) had persistent bacteremia after treatment with a glycopeptide antibiotic. In 52 of the 90 cases, patients continued treatment with a glycopeptide. The remaining 38 patients received linezolid. The 2 groups had similar rates of clinical success (74% with linezolid vs. 70% with the glycopeptide). However, the 30-day mortality trended in favour of the former group (11% vs. 25%, P=0.08), as did
S. aureus-related mortality (5% vs. 17%, P=0.08).
Alternative Treatment Choices for VAP
Intravenous (i.v.) colistin exhibits poor penetration into the lung parenchyma, making its use in the treatment of pneumonia controversial. In contrast, inhaled colistin achieves high concentrations in respiratory tissue and avoids AEs associated with systemic therapy. Clinical experience with colistin in non-cystic fibrosis populations is limited.
Florescu et al. performed a meta-analysis of randomized clinical trials to assess colistin’s potential value as a treatment for VAP (abstract 299). The analysis included 6 studies and 359 patients of whom 186 were treated with colistin (i.v. or inhaled) and 173 with other antibiotics. Overall, outcomes with colistin did not differ significantly from those achieved with the comparator drugs (OR 1.14 for clinical response). Analysis of colistin outcomes by route of administration showed no difference between a nebulized vs. an i.v. formulation.
Investigators in a randomized, phase IV clinical trial performed a post hoc analysis to compare outcomes with linezolid and vancomycin in patients with VAP and culture-proven MRSA (abstract 572). Specifically, they analyzed the time to resolution of signs and symptoms with each agent. The results showed non-significant higher rates of resolution with linezolid on day 3 and day 6, including râles, pulmonary consolidation and hypoxemia. Thereafter, rates of symptom resolution with vancomycin began to increase and equaled or surpassed resolution rates in the linezolid arm at EOT and EOS. None of these differences was statistically significant. At EOT, significantly more patients had normalization of breath sounds (P=0.003) and normalization of chest X-ray (88.5% vs. 70.1%, P=0.0005) (Table 2).
Table 2.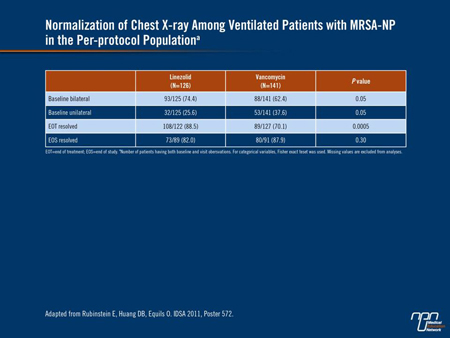
Looking to the Future
Development of new therapies for MRSA pneumonia continues and several new compounds could become available in the near future. Among agents showing potential are PTK 0796, the first member of a new class of broad-spectrum antibiotics known as aminomethylcyclines with oral and i.v. formulations; fusidic acid, an orally active bacteriostatic antibiotic developed for gram-positive pathogens, including MRSA strains associated with HAP; LCB01-0371, an oxazolidinone with broad-spectrum activity, has demonstrated efficacy comparable to or better than several agents already on the market; and LTX-109, a synthetic peptidomimetic that has membrane-lysing activity and whose development includes a topical formulation for eradication of nasal colonization.
The growing problem posed by resistant organisms has created an urgent need for more effective therapies. The IDSA has launched an initiative to encourage the development of 10 new antibiotic agents by 2020. Called the “10 by 20 Initiative,” the program was developed to encourage greater collaboration among academic medicine, industry and government to bring effective new antibiotics to the market more promptly.
Summary
MRSA pneumonia poses a major public health problem, exacerbated by pathogenic resistance, fostered in large part by overuse and inappropriate use of antibiotics. Vancomycin, for years the standard of care for treatment of resistant organisms, including MRSA, has compiled a suboptimal clinical record. Some newer agents have shown promise as effective alternatives to vancomycin. However, more options are needed, as problems with resistant organisms persist despite the introduction of new agents in recent years. Several drugs in development have shown promise as potentially effective therapies for MRSA and other resistant organisms. However, the search for more effective therapies must continue, along with development of effective strategies for prevention and to increase appropriate use of available antibiotics.