Reports
Rare Kidney Disease in the Time of COVID: Update on Atypical Hemolytic Uremic Syndrome (aHUS)
This report is based on medical evidence presented at sanctioned medical congress, from peer reviewed literature or opinion provided by a qualified healthcare practitioner. The consumption of the information contained within this report is intended for qualified Canadian healthcare practitioners only.
PRIORITY PRESS - Kidney Week 2021 (American Society of Nephrology)
Online / November 4–7, 2021
Online – Despite its rarity, atypical hemolytic uremic syndrome (aHUS) featured prominently in this year’s virtual Kidney Week – including the second reported case worldwide of aHUS due to COVID-19. Presenters explored the challenging diagnosis of aHUS, in which kidney damage is central, through successful cases and biomarker data. Optimisation of Canadian treatment standard, eculizumab, was also featured, along with trials of new therapies.
Chief Medical Editor: Dr. Léna Coïc, Montréal, Quebec
Dr. Abraham presented a poster1 on an unusual manifestation of COVID-19: aHUS. Dr. Abraham’s case report was only the second such report worldwide. aHUS, a rare form of thrombotic microangiopathy (TMA), is caused by dysregulation and overactivation of the alternative pathway of the complement system, leading to a triad of Coombs-negative hemolytic anemia, thrombocytopenia and acute kidney failure. aHUS has a mortality rate of 25% and half of all patients progress to end-stage renal disease.1 Health Canada approved the first therapy for aHUS in March, 2013: C5-inhibitor eculizumab.
Despite its rarity, aHUS was well covered during Kidney Week 2021, with three sessions and 22 posters reflecting the intense interest in diagnosis and new therapies. “The kidneys seem to take the brunt of the damage in aHUS,” summed up an industry speaker during a poster talk on C5-inhibitor ravulizumab (which is not approved in Canada for aHUS).
Challenges of Diagnosis: Biomarkers
Eleven of the 22 posters covered the difficulties of diagnosing aHUS. “Clearly, better biomarkers are needed for this disease,” said Dr. Caroline Duineveld of Radboud University Medical Center in the Netherlands, in an interview about her center’s challenges with identifying aHUS quickly enough following kidney transplantation2.
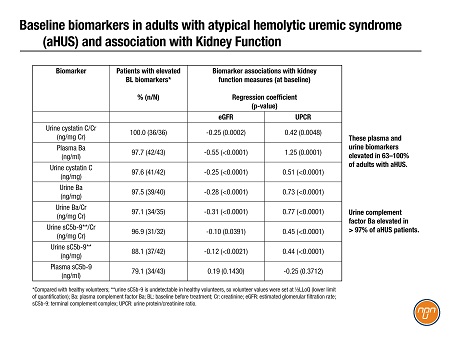
Biomarkers are now starting to emerge from sub-analyses of pivotal trials for new drugs. An industry team shared an exploratory analysis3 of diagnostic and prognostic biomarkers of aHUS garnered from a phase 3 study of ravulizumab. Eight urine and plasma biomarkers were elevated in 63–100% of adults with aHUS (Table 1); urine complement factor Ba levels were higher in all but one patient (N=32) and significantly associated with clinical improvements after treatment (data not shown).
Table 1. Biomarkers in Adults with Atypical Hemolytic Uremic Syndrome aHUS and Association with Kidney Function1
Challenges of Diagnosis: aHUS Cases
In interviews, Dr. Abraham, Dr. Nicole Wyatt of Brookwood Baptist Health in Birmingham, Alabama, and Dr. Fehlin Stone of Walter Reed National Military Medical Center in Bethesda, Maryland, shared the decision making that led them to identify aHUS in their patients.
Case 1 – Dr. Abraham: 55-year-old woman with acute kidney injury (AKI) due to COVID-191
Altered mental status; shortness of breath; creatinine (Cr) 4.8 mg/dL; Hb 8.9 g/dL; platelets 20,000/µL; on smear, large number of schistocytes and thrombocytopenia; haptoglobin 28 mg/dL; reticulocytes: 3.8%; PLASMIC score: 6; no significant improvement with plasma-exchange therapy (PEX) and steroids; ADAMTS13-level: 65%, ruling out a diagnosis of thrombotic thrombocytopenic purpura (TTP). The patient responded “remarkably well (within days)” to eculizumab treatment but progressed to end-stage renal disease.
Dr. Abraham commented: “With ADAMTS13 returning within acceptable limits makes it much more likely to be [aHUS].”
Case 2 – Dr. Wyatt: 20-year old, 30 weeks into first pregnancy4
Severe abdominal pain, diffuse vaginal bleeding; cesarian section revealed placental abruption and fetal demise; maximum serum Cr 8.43 mg/dL; Hb 5.5 g/dL; platelets 15,000/µL, lactate dehydrogenase 9051 U/L; schistocytes on smear; worsening symptoms despite renal-replacement therapy, daily plasma exchange and steroids; ADAMTS13-level: 83%. After 1 week of eculizumab, “hematological parameters normalized with evidence of renal recovery” and patient remained in remission after
6 months on eculizumab. Genetic testing: rare variant in THBD (thrombomodulin gene).
Dr. Wyatt commented: “The leading differential in our case was originally HELLP syndrome vs TTP…the diagnosis of HELLP was ruled out when the patient continued to experience clinical deterioration postpartum. TTP was ultimately ruled out with normal ADAMTS13.”
Case 3 – Dr. Stone: “A tricky diagnosis” in a 22-year-old man with hypertension, vision loss and AKI5
BP: 240/140; bilateral Frisén Grade IV papilledema; sCR: 4.9 mg/dL; proteinuria: 4g; platelet count, Hb, LDH, haptoglobin, Factor H auto antibodies, complement, hepatitis/HIV, auto-immune biomarkers, cryoglobins: normal; renal biopsy: >50% glomerular obsolescence and 35% interstitial fibrosis/tubular atrophy and arteriolar indicators “concerning for a subtle TMA.” Genetics: homozygous deletion of CFHR3-CFHR1, confirming complement-mediated TMA. Vision, BP and sCr improved with antihypertensives and C5 inhibitors.
Dr. Stone commented: “What was surprising about this case is just how negative his whole workup was. Almost every single lab I sent off returned as normal. With the biopsy, it could have been thrombotic microangiography, but we didn’t have a clue what type…sending off the Factor H and genetic testing was just to check it off the differential checklist.”
What advice would these physicians give their colleagues?
Dr. Stone advised, “[don’t] freak out, and think through the differentials logically. For this unfortunate patient, every single differential diagnosis was on the table... But it was truly rewarding when I finally had an answer for him with a treatment modality.”
Dr. Wyatt said, “It’s reasonable to initiate complement-blockade therapy while awaiting further workup in a postpartum patient with evidence of TMA and renal failure.” She added, “ It is essential to obtain genetic testing on all suspected PaHUS patients”.
Optimal Use of Eculizumab
Also at the conference were results from CUREiHUS, a Dutch observational study monitoring outcomes of Dutch guidelines that mandate discontinuation of eculizumab in recovered aHUS patients.6 CUREiHUS monitored 21 patients from January, 2016, until October, 2020. After full recovery of TMA parameters – over a treatment duration of 13.6 weeks (range 2.1–43.9) – eculizumab was withdrawn. During follow-up of 80.7 weeks, a relapse occurred in four patients (19.0%); median time to first relapse was 14.3 weeks (7.1–62.0). Relapsed patients were re-treated successfully with eculizumab then the therapy was discontinued again. No clinically relevant predictors of relapse were identified.
In an interview, Dr. Romy Bouwmeester of Radboud University Medical Center suggested that centralized follow-up would have helped monitor patients in CUREiHUS: “To our Canadian colleagues, we do recommend to strive to centralize co-ordination of care for aHUS patients…[and] frequent follow-up visits during the first year after eculizumab discontinuation since the majority of relapses will occur within a year...”
Future Therapies, Canadian Trials
Conference posters also had updates on therapies for aHUS not yet available in Canada.
Crovalimab is a self-injected investigational C5-inhibitor. At the conference, researchers from Roche and academic institutions in Europe, Asia and the U.S. presented two phase-3 trials to evaluate crovalimab in children (COMMUTE-p; NCT04958265) and adolescents/adults (COMMUTE-a; NCT04861259).7 The pediatric study started recruiting at CHU Sainte-Justine in Montreal on November 20; the adult study on October 22 at 68 global sites, including Vancouver General Hospital, St. Michael’s Hospital and Toronto General Hospital.
At the conference, Novartis researchers and academic partners presented their plans for APPELHUS (NCT04889430), a phase 3 single-arm, open-label study of investigational oral factor-B inhibitor, iptacopan.8 The study opened on November 8, hoping to recruit 50 adults in Austria, Greece and Japan.
Alexion AstraZeneca scientists presented an oral poster on the design of a phase-3, randomized, double-blind, placebo-controlled study of C5-inhibitor ravulizumab for adults with thrombotic microangiopathy associated with a trigger.9 The global study (NCT04743804) started in April, 2021.
Conclusions
Kidney damage is a central feature of aHUS and early diagnosis is essential. Although aHUS remains largely a challenging diagnosis of exclusion, biomarkers such as urine complement factor Ba and genetics may be helpful. Resolution of symptoms on treatment with eculizumab often confirms the diagnosis. Potential future therapies include ravulizumab, crovalimab and iptacopan.
References:
1. Abraham RR, Akabane H. Poster PO0125 presented at Kidney Week, Nov 4–7, 2021.
2. Duineveld C, et al. Poster PO1550 ibid.
3. Cammett TJ, et al. Poster PO0239 ibid.
4. Wyatt N, et al. Poster PO2229 ibid.
5. Stone F, et al. Poster PO0293 ibid.
6. Bouwmeester RN, et al. Poster PO1655 ibid.
7. Sheerin NS, et al. Poster INFO07 ibid.
8. Kavanagh DG, et al. Poster INFO30 ibid.
9. Zeesham K, et al. Poster INFO27 ibid.