Reports
Treating Lower Urinary Tract Symptoms in Men with Benign Prostatic Hypertrophy
This report is based on medical evidence presented at sanctioned medical congress, from peer reviewed literature or opinion provided by a qualified healthcare practitioner. The consumption of the information contained within this report is intended for qualified Canadian healthcare practitioners only.
PRIORITY PRESS - 27th Annual Congress of the European Association of Urology (EAU)
Paris, France / February 24-28, 2012
Paris - Lower urinary tract symptoms (LUTS) associated with benign prostatic hypertrophy (BPH) are common in middle-aged or older men. Agents that inhibit α1-adrenoceptors (α-blockers) are the therapy of choice recommended by international guidelines. For appropriate management of LUTS/BPH, they are administered as monotherapy or in combination for patients with concomitant conditions. All α-blockers increase urinary flow and decrease residual bladder volume but they have different levels of selectivity for α1-receptors and may differ in terms of tolerability and possible drug interactions. The implications of these differences for clinical management of BPH-related LUTS were discussed here at the EAU congress in the light of recent research findings.
Chief Medical Editor: Dr. Léna Coïc, Montréal, Quebec
It is estimated that one-half of all men with benign prostatic hypertrophy (BPH) experience lower urinary tract symptoms (LUTS) of which urinary hesitancy, weak stream and nocturia are the most commonly reported. Given the progressive nature of BPH, patients with LUTS will deteriorate over time and their condition can lead to acute urinary retention (AUR) (Can Urol Assoc J 2009;3(3 suppl 2):S92-S100).
Treatment with α-blockers has been shown to relieve BPH-related symptoms and improve urinary flow. They are believed to act by inducing smooth muscle relaxation in the bladder neck, prostate and prostatic capsule through antagonism of α1-adrenergic receptors. Most of these receptors appear to belong to the α1A-subtype. Early α-blockers that were nonselective for adrenoceptor subtypes have been associated with blood pressure (BP)-related adverse effects, such as orthostatic hypotension, that may be attributed at least in part to the blockade of α1B-adrenoceptors in arterial vessels (Pharmacother 2010;30(12):1303-12). The more recent and selective α-blockers tamsulosin and alfuzosin have demonstrated fewer effects on BP.
Overview of Alpha-receptor Selectivity
While tamsulosin has demonstrated efficacy in randomized controlled trials, an agent with greater selectivity like silodosin could confer similar or greater efficacy with fewer adverse events. Characteristics of this novel α-blocker were a point of discussion during this year’s EAU.
Tamsulosin and silodosin have a high affinity for α1A-receptors (9.70 and 10.44 pKi, respectively), but silodosin has a markedly greater preferential selectivity ratio for α1A-receptors vs. the α1B-receptor (593 vs. 6.3) and α1D-receptors (56.7 vs. 0.2). Consequently, it has been suggested that silodosin may be more effective in treating urinary symptoms with fewer side effects on the cardiovascular (CV) system, explained Dr. Christian Gratzke, Ludwig-Maximilians-University, Munich, Germany. However, it may also affect ejaculatory function, since α1A-receptors are essential for physiological contraction of the vas deferens and hence for sperm delivery from the testes to the urethra. The novel α-blocker also has a lower affinity for α1B-receptors (7.67 vs. 8.90). “This may be important, since the α1B-receptor is involved in the regulation of BP,” Dr. Gratzke noted.
CV Safety
In healthy young men (18-45 years), silodosin 8 or 24 mg given for 5 days had no statistically significant effect on QT interval. There were no clinically relevant or statistically significant effects on systolic or diastolic BP or supine heart rate in comparison with placebo (Clin Pharmacol Ther 2010;87:609-13).
In a pooled analysis of European, Japanese and US efficacy trials, Dr. Gratzke reported that the safety of the novel α-blocker “was found to be similar to placebo, with a low frequency of CV adverse events reported.” European trials also showed no increase in dizziness, headache or orthostatic episodes. Prof. Karl-Dietrich Sievert, University of Tübingen, Germany, noted that the novel agent “is a good option in BPH/LUTS patients with CV comorbidity.”
Long-term Safety
A European placebo-controlled trial randomized 955 men to silodosin 8 mg, tamsulosin 0.4 mg or placebo given over 12 weeks. The novel agent had a rapid onset of action and with numerically greater changes from baseline, proved non-inferior to tamsulosin (Eur Urol 2011;59:342-52). Prof. Teuvo L.J. Tammela, Tampere University Hospital, Finland, presented data from a 40-week, open-label extension phase of this study. A total of 500 men entered the extension phase and received silodosin 8 mg q.d. Findings showed that in men with LUTS suggestive of BPH, active treatment is effective and well tolerated over the long term.
Table 1.
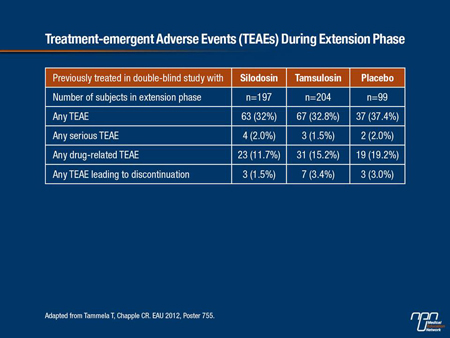
During the extension phase, at least 1 treatment-emergent adverse event (TEAE) was reported by 33.4% of subjects (Table 1).
Treatment-related AEs were reported in 14.6% of patients, 1.8% reported a serious AE and 2.6% experienced an AE that led to discontinuation. Dizziness occurred in 0.8% of participants. No cases of orthostatic hypotension were reported. There were no clinically significant findings in vital signs, laboratory parameters or ECGs. The most frequently reported TEAE was ejaculation disorders (9.0%) followed by influenza (2.8%).
Although abnormal ejaculation is a class effect of a1-blockers, “it is rarely serious enough to prompt patients to withdraw from treatment,” stated Dr. Alberto Briganti, University Vita-Salute San Raffaele, Milan, Italy. In prospective randomized trials with silodosin, the combined prevalence of ejaculatory changes is about 28%. From epidemiological longitudinal studies, ejaculatory change is already present in many older men long before they start treatment for urinary symptoms, “so those elderly patients are less likely to perceive this secondary effect,” he remarked.
Retrograde ejaculation (RE) appears to be an indicator of higher efficacy of silodosin, Dr. Briganti noted. This was confirmed by subanalysis of data from phase III studies of BPH symptom treatment over 12 weeks. Of the 466 patients receiving the active therapy,
28% reported RE compared with 0.9% of placebo-treated patients. For patients with RE, the odds of achieving highest improvement in urinary symptoms (International Prostate Symptom Score >3) and urinary flow rates (Qmax ≥3 mL/s) were 1.75 times those for patients without RE (P<0.012) (Prostate Cancer Prostatic Dis 2011;14:143-8).
“Patients should be counselled about the potential onset of ejaculatory change and its positive clinical implication,” Dr. Briganti recommended. Prof. Tammela pointed out that abnormal ejaculation will resolve within 3-4 days of stopping treatment.
Combination Therapy
For the treatment of erectile dysfunction, it is recommended that patients on a-blockers should be hemodynamically stable before starting treatment with a phosphodiesterase type 5 (PDE-5) inhibitor to minimize the potential orthostatic hypotensive effect. However, Dr. Gratzke noted that silodosin lacks clinically relevant interaction with sildenafil or tadalafil. In combination with tadalafil, it appears to exhibit synergistic inhibitory effects on nerve-mediated contractions of isolated prostate tissue.
The lack of pharmacodynamic interaction with sildenafil and tadalafil was demonstrated in a small study of 22 healthy men (Urology 2010;75:520-5). Co-administration of the 8-mg dose for 21 days with a single dose of sildenafil 100 mg or tadalafil 20 mg on days 7, 14 and 21 caused a small but statistically significant reduction in BP but no significant orthostatic symptoms. The number of post-dose positive orthostatic tests (decrease in systolic BP >30 mm Hg, decrease in diastolic BP >20 mm Hg, increase in heart rate >20 bpm or presence of orthostatic symptoms) was similar for all treatment groups including placebo.
New pharmacological data presented at the congress by researchers from San Raffaele University showed that silodosin and tadalafil concentration-dependently reduced nerve-induced contractions of human prostate preparations. “There appeared to be an additive effect of silodosin and tadalafil combined on prostatic smooth muscle relaxation approximately 100-fold greater than with each drug alone,” Dr. Gratzke told delegates. “If confirmed in a clinical setting, it may allow for optimization of dose and activity and may avoid side effects.”
Dr. Briganti pointed out that it is very common in clinical practice to give both drugs together “since there is no significant interaction between the 2 at any point in time.”
Summary
The high selectivity and affinity of silodosin to α1A-receptors may confer a more favourable side-effect profile relative to previous α-blockers. Notably, it appears to have little effect on BP and has demonstrated a relatively safe CV profile in clinical trials. These are important characteristics in an aging population where comorbidities increase.