Reports
VEGF Inhibition in Colorectal Cancer: Changing the Treatment Paradigm
This report is based on medical evidence presented at sanctioned medical congress, from peer reviewed literature or opinion provided by a qualified healthcare practitioner. The consumption of the information contained within this report is intended for qualified Canadian healthcare practitioners only.
ABSTRACTS in PERSPECTIVE - Based on presentations from the 48th Annual Meeting of the American Society of Clinical Oncology
Chicago, Illinois / June 1-5, 2012
EDITORIAL OVERVIEW:
Prof. Dirk Arnold, MD
Director, Hubertus Wald Tumor Center,
University Cancer Center Hamburg,
University Hospital Eppendorf,
Hamburg, Germany
Hagen Kennecke, MD, MHA, FRCPC
Medical Oncologist and Clinician Researcher,
British Columbia Cancer Agency,
Associate Professor,
University of British Columbia
Vancouver, British Columbia
Targeted molecular therapies provide an opportunity to extend survival for patients with unresectable metastatic colorectal cancer (mCRC), a terminal disease process. Fundamentally different from cytotoxic chemotherapies with which they are often combined, the monoclonal antibodies (MAbs) and tyrosine kinase inhibitors (TKIs) used in mCRC are specifically directed against pathways of tumour growth, such as the angiogenic signals that tumours generate to sustain blood supply. While the likelihood of transforming mCRC from a progressive to a non-progressive, lifelong chronic condition is remote at this time, new data continue to encourage pursuit of this concept.
New study data presented at the 2012 ASCO meeting highlighted the role of targeted agents to prolong disease control in mCRC. The major targets with these therapies are vascular endothelial growth factor (VEGF) and epidermal growth factor receptor (EGFR), each of which appears to be important to tumour proliferation. The benefit from targeted therapies is already well established in mCRC and the new data expand indications and have demonstrated survival benefits in multiple lines of therapy while preserving a high degree of quality of life. Evidence for new agents and more ambitious strategies, such as combining MAbs and TKIs, were also explored in new data presented this year.
Re-Introducing an Anti-VEGF with a Second-line Regimen
Of the studies with relevance to clinical practice, the phase III TML 18147 multinational study demonstrated that the recombinant, humanized anti-VEGF MAb bevacizumab demonstrates benefit when continued in combination with a second-line regimen in patients who received bevacizumab with their first-line regimen. Previous studies had already established bevacizumab as a standard of care in first-line therapy for mCRC and in second-line chemotherapy for those not previously treated with bevacizumab. The results confirmed that VEGF inhibition remains an important target even as disease progresses in patients previously treated with bevacizumab in the first-line setting.
The TML 18147 trial randomized 820 mCRC patients who progressed while on, or within a maximum of 3 months after discontinuation of first-line bevacizumab plus oxaliplatin or irinotecan-based chemotherapy to the alternating secondline chemotherapy with or without bevacizumab. The primary end point was overall survival (OS). The survival advantage of bevacizumab translated into a hazard ratio (HR) of 0.81 (0.69-0.94; P=0.0062), independent from selected chemotherapy. The absolute median benefit of about 6 weeks in this aggressive cancer is both statistically and clinically significant.
The results of TML 18147 support the maintained use of anti-angiogenic therapy over multiple lines of therapy. Safety analyses did not show any new safety concerns when bevacizumab was continued with a second-line regimen. Although the rate of grade ≥3 adverse events was slightly increased on bevacizumab (64% vs. 58%), the rate of serious adverse events was slightly less (32% vs. 34%). Neither difference was statistically significant. The rate of treatment discontinuations was 16% and 9% for arms with and without bevacizumab, respectively.
Dual Pathway Inhibition
The multinational phase III DREAM trial, which tested the value of adding the TKI erlotinib (an intracellular inhibitor of the EGF receptor pathway) to bevacizumab, explored the important concept of further improving disease control by inhibiting more proliferative pathways. DREAM was a maintenance study that enrolled 700 unresectable mCRC patients who had responsive or stable disease after induction of 12-24 weeks with FOLFOX, XELOX or FOLFIRI plus bevacizumab. They were randomized to remain on bevacizumab alone or with erlotinib. The primary outcome was progression-free survival (PFS) during the maintenance period, which was significantly improved (HR 0.73; 95% CI, 0.59-0.91; P=0.005) in the arm that received maintenance therapy with both bevacizumab and erlotinib. OS data were not presented due to limited follow-up time.
Study results support the clinical value of dual pathway inhibition for longer disease control. The strategy of this dual pathway inhibition was reasonably well tolerated when employed in combination with chemotherapy. In the DREAM study, the rate of grade ≥3 adverse events was not significantly greater on the bevacizumab/erlotinib combination compared to bevacizumab without erlotinib when both were added to chemotherapy. A relative increase in diarrhea and skin rash was reported for the combination but these were relatively mild in severity. However, the limitations of the study are that no standard arm was used. As a result, both treatment arms must be considered as experimental. Therefore, no definitive conclusion can be drawn.
There were several other studies presented at the 2012 annual meeting of ASCO that also support a potential benefit from maintenance therapy with a targeted agent. In a relatively small but randomized study, the combination of bevacizumab and capecitabine was compared to continuous bevacizumab and XELOX after induction (abstract 3565). For the primary end point of PFS, the experimental arm of bevacizumab and capecitabine was superior (11 vs. 8.3 months; P=0.002) and grade ≥3 adverse events were less frequent (34.4% vs. 48.4%). While the OS advantage was not statistically significant (23.8 vs. 20.2 months; P=0.1), the overall tolerability of this bevacizumab/capecitabine maintenance suggests it may be a useful strategy after first-line treatment.
Whether in front-line, second-line or maintenance regimens, the principle of proliferative pathway inhibition as a strategy for mCRC control is supported by studies with a range of targeted therapies. The phase III CORRECT study tested regorafenib, a multi-TKI that acts on multiple pathways, including VEGF, Kit, Raf and the platelet-derived growth factor receptor (PDGFR). Patients who were enrolled had progressed after all approved standard therapies including bevacizumab. The primary end point in this study of 760 patients randomized in a 2:1 ratio to this multikinase inhibitor or placebo was OS, which was significantly improved (HR 0.77; 95% CI, 0.64-0.94; P=0.0052). The PFS benefit was even greater (HR 0.49; 95% CI, 0.42-0.58; P<0.000001). The benefit was not affected by KRAS mutation status. The most common side effects of grade ≥3 were hand-foot skin reaction (17%), fatigue (10%), hypertension (7%), diarrhea (7%) and rash (6%). As a result of the improved OS in refractory patients without further options, regorafenib may become a standard of care for those patients.
VEGF Remains a Viable Target
New analyses from the phase III VELOUR study, the results of which were initially presented in 2011, also support targeted therapy in the second-line setting. In VELOUR, mCRC patients initiating a second-line FOLFIRI chemotherapy regimen were randomized to receive aflibercept, a fusion protein VEGF Trap that inhibits VEGF-A, VEGF-B and placental growth factor (PGF), or no targeted agent. While the initial report associated aflibercept with a PFS benefit, new data from the trial with longer follow-up have now demonstrated a statistically significant OS benefit (12.06 vs. 13.5 months: HR 0.82; 95% CI, 0.71-0.94; P=0.0032). Moreover, a subgroup analysis that compared OS benefits in the 373 patients previously exposed to bevacizumab with the 853 patients who had not received a prior VEGF inhibitor found consistent trends for benefit. A prespecified subgroup analysis of this study found no evidence of an interaction with prior bevacizumab exposure. To some extent, these data reinforce the TML 18147 study by showing that VEGF remains a viable target as disease advances, even in patients previously exposed to VEGF inhibition. It is also relevant that the incidence of significant adverse events was also comparable when those who had previously received bevacizumab were compared to those who had not.
Not all novel agents targeting the VEGF receptor have been shown to be effective. In a phase III study with a primary end point of OS, 750 previously treated patients with unresectable mCRC were randomized to cetuximab vs. cetuximab plus brivanib, a multikinase inhibitor acting on the VEGF and fibroblast growth factor receptor (FGFR) pathways. There was a positive effect on PFS (HR 0.72; 95% CI, 0.62- 0.84; P<0.0001) but no significant improvement in OS (HR 0.88; 95% CI, 0.74-1.03; P=0.12). Subgroup analysis, such as stratification for age and prior anti-VEGF therapy, also did not reveal any groups with OS benefit.
Summary
The relatively favourable tolerability profile of most but not all agents targeting the VEGF receptor pathway supports the concept of prolonged therapy with a targeted agent in order to extend OS with an adequate quality of life. This is best illustrated in the survival advantage of continued VEGF inhibitor therapy now demonstrated in 2 sequential lines of therapy, an illustration of the fundamental benefit derived from persistent suppression of molecular signals of tumour proliferation. The value of dual targeted therapy in mCRC remains unclear and further follow-up and trials are required to establish both the efficacy and safety of this approach.
ABSTRACT CRA3503
Bevacizumab (BEV) plus chemotherapy (CT) continued beyond first progression in patients with metastatic colorectal cancer (mCRC) previously treated with BEV plus CT: Results of a randomized phase III intergroup study (TML study).
D. Arnold, T. Andre, J. Bennouna, J. Sastre, P. J. Osterlund, R. Greil, E. Van Cutsem, R. Von Moos, I. Reyes-Rivera, B. Bendahmane, S. Kubicka
Background: BEV in combination with fluoropyrimidine-based CT is standard treatment for mCRC in the firstline (1L) and BEV-naïve second-line (2L) settings. This is the first randomized study evaluating the benefit of continuing BEV in combination with standard CT as 2L treatment for patients with mCRC who progressed after receiving a standard BEV-containing regimen in the 1L setting.
Methods: Patients with unresectable, histologically confirmed mCRC who progressed within 3 months after discontinuation of 1L BEV + CT were randomised to 2L fluoropyrimidine-based CT ± BEV (2.5 mg/kg/wk equivalent). Choice of oxaliplatin- or irinotecan-based 2L CT was dependent on the regimen used in 1L (crossover) and included as a stratification variable. The primary endpoint was overall survival (OS); secondary endpoints included progression-free survival (PFS), response rate and safety.
Results: 820 patients were randomized from February 2006 to June 2010 (409 to BEV + CT and 411 to CT alone). Baseline patient and disease characteristics were well balanced between arms. The study met its primary endpoint; median OS was 11.2 months for BEV + CT and 9.8 months for CT (HR=0.81; 95% CI 0.69–0.94; unstratified log-rank test, p=0.0062). Median PFS was 5.7 months for BEV + CT and 4.1 months for CT (HR=0.68; 95% CI 0.59–0.78; unstratified log-rank test, P<0.0001). The response rate was 5.4% for BEV + CT and 3.9% for CT (unstratified Chi-Square Test, P=0.3113). The adverse event profile was consistent with previously reported data for BEV + CT. Compared with historical data from BEV treatment in 1L or 2L mCRC, BEV-related adverse events were not increased when continuing BEV beyond progression.
Conclusion: This is the first randomized study to prospectively investigate the impact of continuing BEV treatment in 2L mCRC for patients who progressed after receiving a BEV-containing regimen in 1L. Our findings demonstrate that BEV + CT (crossed over from 1L regimen) continued beyond progression significantly prolongs OS and PFS in 2L mCRC. Additional analysis (including biomarker evaluation) is ongoing.
Commentary on abstract CRA3503
The TML 18147 study demonstrated that the addition of bevacizumab to second-line therapy for mCRC in patients who already received bevacizumab with their first-line regimen improves OS. The survival benefit provides a basis for declaring this strategy as a potential new standard of care. These data also reinforce other evidence that inhibition of the VEGF pathway remains a viable target for slowing disease progression over the course of disease regardless of whether VEGF has been inhibited before. The favourable tolerability of bevacizumab, which demonstrated a side-effect profile in the second-line setting that was comparable to what has been previously reported in first-line therapy, enhances its suitability as a strategy for extending mCRC control. The persistent benefit from inhibition of VEGF advances the goal of employing targeted therapies for long-term control of advanced mCRC. As opposed to a strategy of employing cytotoxic agents to eradicate malignancy, VEGF inhibition appears to extend life by delaying progression.
Questions and answers with Prof. Dirk Arnold, University Hospital Eppendorf, Hamburg, Germany
Q: Do the results of this study change the treatment paradigm in mCRC?
A: VEGF is both an early and persistent promoter of angiogenesis. While there have been previous observational studies suggesting bevacizumab added to second- and also third-line chemotherapy correlates with prolonged survival, this is the first multicentre randomized study to demonstrate that bevacizumab should be included in second-line regimens in patients being pretreated with a first-line bevacizumab-containing combination.
Q: One of the advantages of targeted therapies is that they are typically better tolerated than cytotoxic agents, but did patients in this second-line setting continue to tolerate bevacizumab to the same degree as when it is used first-line?
A: The adverse-event profile of bevacizumab in this study was consistent with what has been reported previously in the first-line setting. Furthermore, continued application of bevacizumab did not affect the chemotherapy-related toxicity; this was similar in both arms. The favourable tolerability of bevacizumab is important because it suggests that the survival advantage can be achieved with good preservation of quality of life.
Q: What are the implications of this study and does this study have any relevance to the role of bevacizumab as a maintenance treatment?
A: While these data support bevacizumab as a new second-line treatment option for prolonging control of advanced unresectable colon cancer, the findings also indicate a potential new model for employing a targeted therapy through multiple lines of treatment. This same approach is currently being investigated in other tumour types. These data cannot be directly interpreted as providing support for bevacizumab as a maintenance therapy, but they do demonstrate that bevacizumab is active when re-introduced with a second-line regimen.
ABSTRACT LBA3500
Bevacizumab (Bev) with or without erlotinib as maintenance therapy, following induction first-line chemotherapy plus Bev, in patients (pts) with metastatic colorectal cancer (mCRC): Efficacy and safety results of the International GERCOR DREAM phase III trial.
C. Tournigand, B. Samson, W. Scheithauer, G. Lledo, F. Viret, T. Andre, J.-F. Ramée, N. Tubiana-Mathieu, J. Dauba, O. Dupuis, Y. Rinaldi, M. Mabro, N. Aucoin, A. Khalil, J. Latreille, C. Louvet, D. Brusquant, F. Bonnetain, B. Chibaudel, A. De Gramont
Background: Therapy targeting VEGF or EGFR demonstrated clinical activity in combination with chemotherapy (CT) in mCRC but monoclonal antibodies cannot be associated. The DREAM trial compares a maintenance therapy (MT) with bev +/- EGFR tyrosine kinase inhibitor erlotinib (E) after a first-line Bev-based induction therapy (IT) in pts with mCRC.
Methods: Pts with previously untreated and unresectable mCRC were eligible. After a Bev-based IT with FOLFOX or XELOX or FOLFIRI, pts without disease progression were randomized to MT between Bev alone (Bev 7.5 mg/kg q3w; arm A) or Bev+E (B 7.5 mg/kg q3w, E 150 mg/day continuously; arm B). Pts were treated until progression or unacceptable toxicity. The primary endpoint was PFS on MT.
Results: The study enrolled 700 pts from 01/2007 to 11/2011 in 3 countries (France, Canada, Austria). 446 (63.7%) pts were randomized for MT (arm A, N=224; arm B, N=222). Among the 446 randomized pts, IT regimen was FOLFOX-Bev in 265 pts (59.4%), XELOX-Bev in 135 pts (30.3%), and FOLFIRI-Bev in 46 pts (10.3%). Baseline characteristics of randomized pts were (arm A/B): ECOG PS 0, 60% in both arms; normal LDH level 47%/49%; normal alkaline phosphatase level 48%/50%; synchronous metastasis 83%/82%. The median no of MT cycles was 6 in both arms. With a median follow-up of 31.0 months, 327 PFS events were observed. Median MT-PFS were 4.6 m in arm A vs 5.8 m in arm B (HR 0.73 [95%CI: 0.59-0.91], P=.005). Median PFS from inclusion were 9.2 m vs 10.2 m. During MT, in arm A vs arm B, grade 3-4 diarrhea (<1% vs 9%) and grade 3 skin toxicity (0% vs 19%) were the main differences in toxicity. Severe adverse events from randomization related to B or E were 6 in arm A and 7 in arm B. Overall survival is not mature.
Conclusions: The addition of erlotinib to bevacizumab after induction therapy significantly improves the duration of maintenance PFS, following induction with first-line chemotherapy plus bevacizumab, in patients with unresectable metastatic colorectal cancer.
Commentary on abstract LBA3500
This latebreaking study is among the first to suggest that dual targeted therapy as a maintenance therapy improves PFS in mCRC following induction treatment with combination chemotherapy plus bevacizumab. Therapy was relatively well tolerated. Previous studies of combination cetuximab, which also inhibits EGFR but acts extracellularly, with bevacizumab plus combination therapy produced an unacceptable rate of adverse events. OS data is awaited pending further follow-up. The study is limited by the lack of a standard therapy arm. In light of previous studies demonstrating the value of maintenance bevacizumab in combination with 5-Fluorouracil chemotherapy, future studies to compare bevacizumab alone versus combination therapy with erlotinib or 5-FU may be required.
Questions and answers with Prof. Christophe Tournigand, CHU Saint-Antoine, Paris, France
Q: Benefits in mCRC have been associated with both VEGF and EGFR inhibitors. Is there any sense that one is more important than the other?
A: The more important question may not be which signal is more important for tumour growth but whether inhibiting both pathways limits the crosstalk between signalling systems that allows tumour growth even when one pathway is blocked. There is a great deal of preclinical data showing that simultaneous inhibition of both signalling systems may provide a synergistic effect relative to the anticipated additive effect of inhibiting these pathways. Although the combination of bevacizumab and cetuximab proved to be too toxic, the tolerability of the combination of bevacizumab and erlotinib is encouraging.
Q: Relative to bevacizumab alone, adverse events were increased with the addition of erlotinib. Could you describe these events in regard to quality of life?
A: We did not report quality of life data, but there was a low risk and no significant differences in many of the adverse events for which there might be the most concern, such as hypertension or bleeding. Diarrhea was more common in all grades, but only 9% of those on the combination had grade 3 diarrhea and none had grade 4. Grade 3 skin toxicity was observed in 19% of patients on the combination and grade 4 skin toxicity was observed in 1% vs. 0% of those on bevacizumab alone, but the combination was well tolerated in most patients.
Q: Should the combination of bevacizumab and erlotinib be considered a standard of care for maintenance therapy after an induction regimen containing bevacizumab in mCRC?
A: These results provide a clinical rationale for double inhibition of VEGF and EGFR, but we need longer follow-up to evaluate whether this combination has an impact on survival. These data further support the role of bevacizumab as a maintenance therapy in mCRC, but there are other considerations, including quality of life, that will also need to be considered in the context of cost when considering routine use of bevacizumab plus erlotinib as maintenance in all mCRC patients. However, the significant increase in PFS with dual inhibition is an important finding.
ABSTRACT 3565
Bevacizumab (BEV) plus capecitabine as maintenance therapy after initial treatment with BEV plus XELOX in previously untreated patients (pts) with metastatic colorectal cancer (mCRC): Mature data from STOP and GO, a phase III, randomized, multicenter study.
S. Yalcin, R. Uslu, F. Dane, U. Yilmaz, N. Zengin, E. Buyukunal, S. Buyukberber, C. Camci, O. Sencan, S. Kilickap, F. Ozdener, D. Cevik
Background: Colorectal cancer is one of the most frequent malignancies, second after breast cancer in women and third after lung cancer and prostate cancer in men. The aim of this study was to evaluate and compare the progression-free survival (PFS) between two arms: Arm A is a combination of BEV + XELOX; Arm B is a combination of BEV + XELOX for 6 cycles followed by maintenance BEV + capecitabine as firstline therapy in mCRC.
Methods: BEV (7.5 mg/kg) + XELOX (capecitabine 1000 mg/m2 bid d1–14 + oxaliplatin 130 mg/m2 d1 q3w) were administered until progression (Arm A) or 6 cycles of BEV + XELOX followed by BEV + capecitabine were administered until progression (Arm B). PFS was the primary endpoint; secondary endpoints included overall survival (OS), objective response rate (ORR), and safety. A sample size of 118 pts was required to detect with 80% power an increase of 1.5 months in median PFS between two arms with a standard deviation of 3.9 months and significance level of 0.05 (10% drop-out rate).
Results: A total of 123 pts were randomized. Demographic characteristics were balanced between the arms. Median treatment period was 7.5 (range 0.5–13.9) and 8.1 (range 0.1–20.7) months in Arms A and B, respectively. There was a statistically significant difference in median PFS between arms, although there was no significant difference in ORR and OS (see table). Tolerability was acceptable in both arms with the following grade 3/4 adverse events (AEs): Arm A 48.4%; Arm B 34.4% (P=0.116). Grade 3/4 diarrhoea occurred in 9.7% vs. 3.3%, weakness in 8.1% vs. 8.2%, hand-foot syndrome in 3.2% vs. 1.6%, and neuropathy in 4.8% vs. 3.3% of pts in Arms A and B, respectively.
Conclusions: These findings suggest that maintenance therapy with BEV + capecitabine following induction with 6 cycles of BEV + XELOX may be superior to continuous BEV + XELOX until progression inpts with previously untreated mCRC.
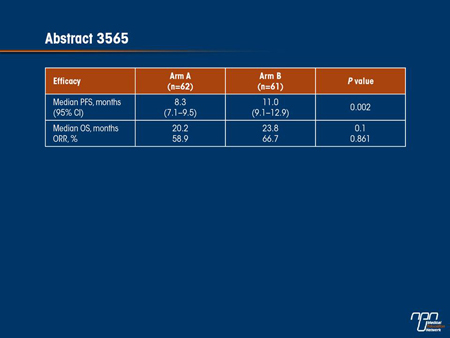
Commentary on abstract 3565
Due to the fact that most or all mCRC patients will relapse during maintenance therapy, there is still debate about whether 6 cycles of induction is sufficient or if longer treatment should be considered. In this study, called STOP and GO, continuous treatment with XELOX plus bevacizumab (on a 3-week cycle) was compared to capecitabine plus bevacizumab after both arms had received an initial 6 cycles of XELOX with bevacizumab. The primary outcome was PFS. The ability of the 2-drug arm to provide a significantly longer PFS (11.0 vs. 8.3 months; P=0.002) than continuous XELOX/bevacizumab is particularly remarkable because the rate of grade ≥3 adverse events was significantly lower. In fact, no grade 3 or 4 adverse event, except for hypertension (3.3% vs. 1.6%), occurred more frequently on the bevacizumab/capecitabine combination and most - including fatigue (6.6% vs. 16.1%), diarrhea (3.3% vs. 11.3%), anorexia (3.3% vs. 11.3%) and neuropathy (1.6% vs. 8.1%) - were observed at a much lower rate. Although both regimens were safe, the greater tolerability of bevacizumab plus capecitabine favours this strategy as a maintenance regimen to prolong PFS. While this specific trial is too small to change current practice, similar but larger studies now underway, such as MACRO, CAIRO-3 and AIO CRC 0207, are expected to provide more definitive conclusions within the next 2 years.
Questions and answers with Dr. Suayib Yalcin, Hacettepe University Institute of Oncology, Ankara, Turkey
Q: It seems counter-intuitive that the simpler, better tolerated regimen provided better PFS than a more aggressive regimen. What is the explanation?
A: One likely explanation is the greater tolerability for the 2-drug regimen. The median treatment period was several weeks shorter in the group that remained on continuous XELOX [7.5 vs. 8.1 months]. The rate of objective responses was also somewhat higher in those who received bevacizumab and capecitabine relative to XELOX [66.7% vs. 59%], although the difference did not reach statistical significance. Even though the difference in PFS was significant, it still may be more appropriate to call bevacizumab and capecitabine at least as effective as continuous XELOX plus bevacizumab after 6 cycles of induction.
Q: But given the better tolerability of bevacizumab plus capecitabine, wouldn’t this regimen be preferred over XELOX plus bevacizumab?
A: On the basis of these data, maintenance therapy with bevacizumab plus capecitabine can be considered an appropriate option following induction with bevacizumab plus XELOX. Continuous XELOX plus bevacizumab after a 6-cycle induction is not a standard of treatment now, but there is increasing interest in some form of maintenance therapy in mCRC, and this regimen demonstrated activity and acceptable tolerability.
Q: How does one judge whether the improvement in PFS warrants the risk of adverse events, which one can presume occur at a higher rate than no therapy after the initial induction?
A: This will differ among patients in regard to their willingness to accept side effects or even particular side effects. However, no grade 3 side effects except for fatigue was observed in more than 5% of patients on maintenance with bevacizumab and capecitabine, and the improvement in PFS was not only substantial in terms of the median measured in months but also in the proportion of patients who remained free of progression at time points out to 36 months. While the difference at 36 months was small (4.6% vs. 1.8%), the proportion who remained progression-free at 12 months was more than twice as great (43.3% vs. 21.6%).
ABSTRACT 3502
Phase III CORRECT trial of regorafenib in metastatic colorectal cancer (mCRC).
E. Van Cutsem, A. F. Sobrero, S. Siena, A. Falcone, M. Ychou, Y. Humblet, O. Bouche, L. Mineur, C. Barone, A. Adenis, J. Tabernero
Background: Regorafenib (REG) is an oral multi-kinase inhibitor. The CORRECT trial was conducted to evaluate REG in patients (pts) with mCRC who had progressed after all approved standard therapies.
Methods: Enrollment criteria included documented mCRC and progression during or ≤3 months after last standard therapy. Pts were randomized 2:1 to receive best supportive care plus either REG (160 mg od po, 3 wks on/1 wk off) or placebo (PL). The primary endpoint was overall survival (OS). Secondary endpoints included progression-free survival (PFS), overall response rate, disease control rate, safety and quality of life (QoL). Efficacy analyses across prespecified subgroups were evaluated using univariate Cox regression.
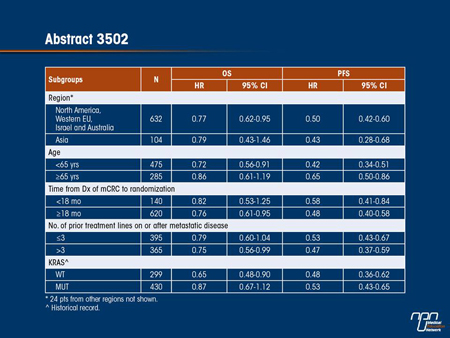
Results: 760 pts were randomized (REG: 505; PL: 255). The OS primary endpoint was met at a preplanned interim analysis. OS and PFS were significantly improved in REG arm compared to PL arm: hazard ratio (HR) for OS 0.77 (95% CI 0.64-0.94, 1-sided P=0.0052), median OS 6.4 vs 5.0 mos; HR for PFS 0.49 (95% CI 0.42-0.58, 1-sided P<0.000001), median PFS 1.9 vs 1.7 mos. Comparable OS and PFS benefits were observed in exploratory subgroup analyses by region, age, time from diagnosis of mCRC to randomization, prior lines of treatment, and KRAS status (shown in table). The most common grade 3+ AEs related to REG were hand-foot skin reaction (16.6%), fatigue (9.6%), hypertension (7.2%), diarrhea (7.2%) and rash/ desquamation (5.8%). QoL data will be presented.
Conclusions: REG demonstrated statistically significant improvement in OS and PFS over PL, as well as comparable efficacy benefits across pt subgroups analyzed.
Commentary on abstract 3502
The phase III CORRECT trial tested regorafenib, an oral multikinase inhibitor that has inhibitory activities on several intracellular molecular pathways of proliferation, including VEGF, PDGFR, Kit and RAF. The study was conducted in patients with mCRC who had progressed after all approved standard therapies had failed. The primary end point of OS was met at a preplanned interim analysis when the HR was 0.77 (95% CI, 0.64-0.94; P=0.0052). A greater PFS benefit was documented (HR 0.49; 95% CI, 0.42- 0.58; P<0.000001). When stratified by a number of variables, including KRAS status, number of prior treatment lines and age, the relative benefits for both OS and PFS were similar. The drug was relatively well tolerated although some grade ≥3 adverse events, including hand-foot reaction (16.6%), fatigue (9.6%), hypertension (7.2%) and diarrhea (7.2%), were associated with this agent. The results are encouraging because of the absence of treatment alternatives in this patient population.
Questions and answers with Prof. Eric Van Cutsem, University Hospital Gasthuisberg, Leuven, Belgium
Q: What do these results mean clinically?
A: They suggest that regorafenib may be a new standard in salvage therapy for mCRC. We now have no such standard even though many of these patients have good performance status. This is a major unmet clinical need.
Q: As a relatively new agent, regorafenib has a limited number of clinical trials in any disease, not just mCRC. Is the term “standard” justified by the results of this study?
A: Regorafenib increased survival as well as PFS, and it did so across all of the prespecified subgroups that we evaluated. Moreover, the tolerability was favourable with manageable side effects. As we have no other options that have demonstrated this type of activity in a phase III study, it is reasonable to conclude that this agent may be a new standard.
Q: What is the potential for benefit from regorafenib earlier in mCRC?
A: The activity from novel agents in advanced cancers generally predicts activity in cancers of the same type at an earlier stage, but there are no data to suggest that this agent will be effective or that it can be safely combined with other active agents, including chemotherapies or MAbs, in first- or second-line mCRC. Certainly, studies at earlier stages will be appropriate based on the activity observed in this trial.
ABSTRACT 3602
Aflibercept versus placebo in combination with FOLFIRI in previously treated metastatic colorectal cancer (mCRC): Mean overall survival (OS) estimation from a phase III trial (VELOUR).
F. Joulain, E. Van Cutsem, S. U. Iqbal, M. Hoyle, C. J. Allegra
Background: VELOUR evaluated the efficacy and safety of the novel fusion protein aflibercept (VEGF Trap) in combination with FOLFIRI in mCRC patients previously treated with oxaliplatin. Median OS showed significant improvement with 12.06 months in placebo arm vs 13.50 months in aflibercept arm (P=0.0032; HR=0.82 [95.34% CI: 0.71 to 0.94]) [Van Cutsem, 2011]. Since survival data in oncology are usually right skewed, median survival is preferred for regulatory purposes. However, mean survival estimation can render a more meaningful estimate for long term benefit of interventions, and be effectively applied to clinical and economic decision support. Estimating mean OS also allows payers to derive an estimation of total costs and outcomes in the population. The purpose of this study was to estimate the mean OS for VELOUR trial.
Methods: During the trial follow-up period, mean OS is not observable and can be estimated by extrapolating the trial Kaplan-Meier curve using a survival function. Several standard parametric distributions were tested: exponential, weibull, lognormal, loglogistic and gompertz. Akaike’s Information Criteria (AIC), Bayesian Information Criteria (BIC) and graphical method were used to evaluate the goodness of fit of the distributions. Models were run by treatment arm separately or combining the two arms and using treatment as covariate to control for variation.
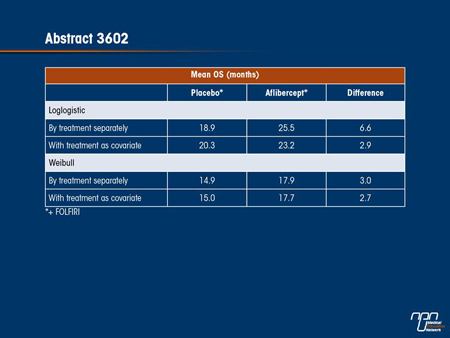
Results: Using AIC/BIC and graphical method, loglogistic function best fit the VELOUR data both with and without treatment as covariate. Weibull distribution is used for sensitivity analysis.
Conclusions: Using loglogistic function, mean OS benefit for aflibercept in combination with FOLFIRI is at least 2.9 months (versus 1.4 months difference in median survival). The results have important implications for clinical and economic decision support.
Commentary on abstract 3602
Careful calculation of the relative survival benefit of treatments used in mCRC, like other cancers in which cure is not an expected outcome, is valuable for informing patients considering treatments that have costs and the potential for adverse events. In the VELOUR study, the median survival advantage of adding the VEGF Trap agent aflibercept to FOLFIRI for second-line treatment of mCRC was approximately 1.5 months (13.5 vs. 12.06 months), producing a significant favourable HR of 0.82 (95% CI, 0.71- 0.94; P=0.0032). The mean survival advantage may also be meaningful for evaluating relative benefit. In this study, mean survival was calculated with several different analytical models in which aflibercept was evaluated both as a stand-alone variable and in relation to FOLFIRI. Based on what the investigators described as the best fit for the data, it was estimated that the mean OS benefit is at least 2.9 months, which is more than double that of the median survival benefit. The investigators suggested that this greater mean survival has important implications for both counselling patients and economic modelling.
Questions and answers with Florence Joulain, MSc, Paris, France
Q: The median is the more commonly reported statistic when presenting outcomes such as survival. Why perform a mean calculation?
A: Some patients benefit much more from a therapy than others and this can be concealed by the median, which reflects the average or middle value and omits the impact of prolonged responses in the subpopulation of good responders. For a patient considering a treatment, a mean response may provide more insight than a median response into the potential for benefit.
Q: Is there a risk that this may appear as data manipulation?
A: In the initial analysis, the median survival benefit was highly statistically and clinically significant, so this was not an effort to improve the outcome. However, it is reasonable to consider both the median and the mean survival because of the different information they provide. It is relevant to patient expectations, and it is useful for estimating the cost-benefit of funding the therapy.
Q: Another sub-study of VELOUR indicated that the survival benefit of aflibercept with FOLFIRI in second-line therapy was observed independent of exposure to bevacizumab in first-line therapy. What does this tell us about VEGF inhibition?
A: We now have 2 sets of data presented at this meeting that suggest that VEGF inhibition remains effective second-line even after it was used first-line. I think the message is that VEGF continues to drive mCRC progression and that inhibition is important probably across lines of therapy.
ABSTRACT 3505
Effects of prior bevacizumab (B) use on outcomes from the VELOUR study: A phase III study of aflibercept (Afl) and FOLFIRI in patients (pts) with metastatic colorectal cancer (mCRC) after failure of an oxaliplatin regimen.
C. J. Allegra, R. Lakomy, J. Tabernero, J. Prausová, P. Ruff, G. Van Hazel, V. M. Moiseyenko, D. R Ferry, J. J McKendrick, E. Van Cutsem
Background: Aflibercept (Afl; also known as VEGF Trap) is a recombinant human fusion protein that acts as a decoy receptor and prevents the interaction of vascular endothelial growth factor (VEGF)-A, VEGF-B, and placental growth factor (PlGF) with their receptors. In the phase III VELOUR study, Afl + FOLFIRI improved overall survival (OS) compared with FOLFIRI + placebo (pbo) in mCRC (ECCO 2011, abstract 6LBA). We report outcomes from a pre-specified subgroup analysis by prior B use.
Methods: Pts with mCRC and progression during or after oxaliplatin were randomized 1:1 to receive either FOLFIRI + pbo or FOLFIRI + Afl 4 mg/kg IV Q2W with stratification by ECOG performance score (PS, 0 v 1 v 2) and prior B. OS and progression-free survival (PFS) in the prior B-treated pts are reported as median estimate and hazard ratios (HRs); 95.34% CI for OS and 95% CI for PFS.
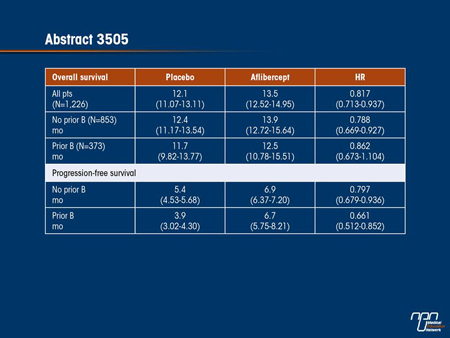
Results: Of the 1226 pts in the overall study, 187 in the pbo and 186 in the Afl group were stratified to prior B. The 2 arms were well balanced: median age 60 yrs; male 58%; PS 0-1 97%; and 55% >1 metastatic organ. Although not powered for survival, Afl produced a consistent trend towards prolonged OS and PFS, regardless of prior B use, with no evidence of interaction (OS, P=0.7231; PFS, P=0.6954). The incidence of treatmentemergent adverse events in the Afl arm was similar in pts with prior B (100%) to those without (98.9%), with a similar incidence of grade 3/4 events (82.5% and 83.9%, respectively).
Conclusions: Results of this pre-specified subgroup analysis indicate that adding Afl to FOLFIRI resulted in a consistent trend of increased OS and PFS, regardless of prior B use. Prior treatment with B did not appear to impact the safety profile of Afl.
Commentary on abstract 3505
Aflibercept inhibits VEGF-A and B and PIGF by acting as a decoy receptor. In the phase III VELOUR study, aflibercept plus FOLFIRI was associated with an improvement in OS and PFS relative to FOLFIRI plus placebo in second-line therapy for mCRC. This new analysis of the VELOUR study evaluated the effect of aflibercept in the subgroup of patients who had previously been treated with bevacizumab. When the 2 groups were compared, the relative advantage of aflibercept persisted irrespective of prior bevacizumab exposure. These results support the use of aflibercept in mCRC patients who have previously received a VEGF inhibitor. Moreover, they contribute additional evidence to the premise that VEGF remains an active promoter of mCRC progression and is a viable target of therapy in second- as well as first-line therapy.
Questions and answers with Dr. Carmen Joseph Allegra, University of Florida, Gainesville
Q: Were these results expected?
A: There is now abundant evidence that VEGF is a persistent signal for tumour growth in mCRC. The VELOUR study demonstrated that the VEGF Trap aflibercept, like the MAb bevacizumab, inhibits mCRC progression. Based on previous studies, prior exposure to bevacizumab was not expected to modify the activity of aflibercept, but it was important to show this in a prospective trial. The results suggested that aflibercept was no more and no less effective in those previously treated with bevacizumab.
Q: Is there any difference in efficacy between bevacizumab and aflibercept?
A: There have been no comparative clinical studies in mCRC, so there is no basis on which to compare either the efficacy or the safety of these 2 agents, but we can say that inhibition of VEGF with either agent not only delays progression but extends survival. It is also notable that we now have several studies suggesting the inhibition of VEGF in a second-line regimen has an important anti-tumour effect in patients who also received a VEGF inhibitor first-line.
Q: This was a subgroup analysis. Does this limit the validity of the results?
A: This was a pre-specified subgroup analysis. A substantial number of mCRC patients now receive bevacizumab first-line and we did not want to exclude these patients. Consequently, we decided to include all patients regardless of prior bevacizumab use, but we did pre-specify our intention to evaluate the effect of prior bevacizumab on outcome. In fact, the bevacizumab and no-bevacizumab groups were well balanced, and the evidence that VEGF inhibition with second-line aflibercept is effective after firstline bevacizumab is fairly compelling.
ABSTRACT 3504
Final analysis of the phase III randomized trial of cetuximab (CET) plus either brivanib alaninate (BRIV) or placebo in patients (pts) with chemotherapy refractory, K-RAS wild-type (WT), metastatic colorectal carcinoma (mCRC): The NCIC Clinical Trials Group and AGITG CO.20 trial.
L. L. Siu, J. D. Shapiro, D. J. Jonker, C. S. Karapetis, J. R. Zalcberg, J. Simes, F. Couture, M. J. Moore, T. J. Price, J. Siddiqui, L. M. Nott, D. Charpentier, W. S. Liauw, M. B. Sawyer, M. Jefford, N. M. Magoski, A. M. Haydon, I. B. Walters, D. Tu, C. J. O’Callaghan
Background: The anti-EGFR monoclonal antibody CET has improved survival in pts with chemotherapy refractory, K-RAS WT mCRC. BRIV is a potent inhibitor of multiple receptor tyrosine kinases including both VEGFR and FGFR. The combination of CET and BRIV targets tumor growth and angiogenesis and demonstrated encouraging activity in an early phase clinical trial.
Methods: Pts with mCRC previously treated with combination chemotherapy were randomized 1:1 to receive CET 400 mg/m2 IV loading dose followed by weekly maintenance of 250 mg/m2 plus either BRIV 800 mg PO daily (Arm A) or placebo (Arm B). Pts may have had 1 prior anti-VEGF, but no prior anti-EGFR therapy. Primary endpoint was overall survival (OS).
Results: From 02/2008 to 02/2011, 750 pts were randomized (376 in Arm A and 374 in Arm B). Demographics: median age=64 (range 27-88); male=64%; ECOG 0:1:2 (%)=32:58:10; >3 prior chemotherapy regimens=92%; prior anti-VEGF therapy=41%; K-RAS WT=97%. Primary analysis was conducted per protocol after 536 deaths were observed, with median OS of 8.8 months in Arm A and 8.1 months in Arm B, hazard ratio (HR)=0.88; 95% CI=0.74 to 1.03; p=0.12. Median progression-free survival (PFS) was 5.0 months in Arm A and 3.4 months in Arm B, HR=0.72; 95% CI=0.62 to 0.84; P<0.0001. Incidence of any ≥grade 3 adverse event (AE) was 78% in Arm A and 53% in Arm B. Time to deterioration of physical function was shorter and global quality of life scores were lower in Arm A vs Arm B. Planned subgroup analyses revealed no statistically difference in treatment effects on OS based on pre-specified factors of age, gender, ECOG and race. Likewise, no difference was detected based on exploratory subgroup analyses of LDH and prior anti-VEGF therapy.
Conclusions: Despite positive effects on PFS, the combination of CET+BRIV did not significantly improve OS in pts with chemotherapy refractory, K-RAS WT mCRC. Final updated results based on 20-25% additional events for a total of nearly 700 deaths, as well as further exploratory subgroup analyses, will be presented.
Commentary on abstract 3504
The concept of inhibiting multiple pathways of tumour cell proliferation is a logical next step in the effort to control growth by blocking molecular signals of proliferation. While there are now several targeted agents that provide the proof that tumour growth can be slowed by targeted therapy, better and more complete inhibition of proliferative signals offers the potential for even greater and more sustained inhibition of tumour growth. In this study, called CO.20, the goal was to inhibit extracellular EGFR signalling through the MAb cetuximab and intracellular signalling through brivanib alaninate, an oral, small-molecule inhibitor of several proliferative pathways, particularly those related to VEGF and fibroblast growth factor receptor (FGFR). Although there was a favourable and highly significant benefit on PFS in favour of the combination (P<0.0001), the study did not meet its primary end point of an OS benefit. Moreover, the PFS benefit was counterbalanced by a higher incidence of grade ≥3 adverse events (78% vs. 53%) and deterioration in physical function and diminished quality of life which occurred earlier in the treatment than in the control arm.
Questions and answers with Dr. Lilllian L. Siu, UHN-Princess Margaret Hospital, Toronto, Ontario
Q: The CO.20 results were disappointing. What previous studies had provided an expectation of efficacy from this combination?
A: A series of studies in xenograft models suggested that there is potent in vivo activity from combining cetuximab inhibition of EGFR, which drives tumour growth, and brivanib alaninate, which targets receptors driving angiogenesis. We also have data from early phase I trials that a full dose of these agents can be combined with acceptable tolerability. There was an expectation from experimental studies of a synergistic effect from combining EGFR and VEGF inhibition, but the primary end point of OS was not met.
Q: Despite the fact that this trial did not show a benefit on the primary end point, the combination was active on the secondary end points of PFS and objective response. Were the quality of life data discouraging?
A: The co-primary quality of life end points was time to deterioration of at least 10 points in physical functioning and global subscales of the EORTC Quality of Life Questionnaire C-30. In both, the placebo arm was favoured. While the median time to a 10-point reduction in global health was only different by about 2 weeks [1.6 vs. 1.1 months; P=0.02), this level of deterioration in physical function was reached in 1.7 months in the arm receiving brivanib alaninate vs. 5.6 months on placebo [P<0.0001].
Q: Were there subgroups that derived more or less benefit from this combination?
A: We are actively conducting biomarker analysis, and I believe that the updated final analysis of this data will empower us [to better understand relative benefits and risks]. The results should be available soon.
ABSTRACT 3614
Efficacy and safety of bevacizumab in metastatic colorectal cancer (mCRC): Pooled analysis from randomized controlled trials (RCTs).
N. C. Tebbutt, H. Hurwitz, F. Kabbinavar, B. J. Giantonio, Z. Guan, L. Mitchell, D. Waterkamp, J. Tabernero
Background: Bevacizumab (BV) with chemotherapy (CT) is a standard treatment for mCRC. This analysis pooled individual patient data from the clinical databases of seven RCTs (phase II or III) of BV in 1st- or 2nd-line treatment to further define clinical outcomes, including within subgroups.
Methods: Patient data were pooled from 1st-line (AVF2107, NO16966, ARTIST, AVF2192, AVF0780, AGITG MAX) and 2nd-line (ECOG E3200) trials. All analyses were based on the intent-to-treat population. Overall and progression-free survival estimates (OS, PFS) were calculated by Kaplan-Meier methods. To assess differences in time to response variables by treatment arm (CT vs BV + CT), stratified random (overall) and fixed (subgroup comparisons) models were used to estimate pooled hazard ratios (HRs) and 95% confidence intervals (CIs), with each study included as a stratum.
Results: Of the 3,763 pooled patients (CT [n=1773]; BV + CT [n=1990]), 58.8% were male, 39.6% were ≥65 years, and 45.7% had an ECOG performance status ≥1. OS and PFS were statistically significantly increased in BV-treated patients vs control patients. Safety data in this pooled analysis were consistent with the profile of BV from the individual studies.
Conclusions: The addition of bevacizumab to CT resulted in statistically significant improvements in survival outcomes for mCRC patients in the overall analysis, with PFS benefit extending across subgroups defined by CT intensity, CT regimen, and KRAS status.
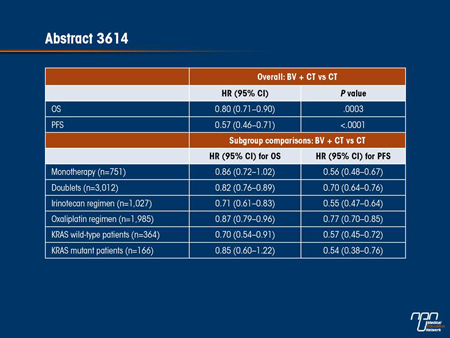
Commentary on abstract 3614
Several meta-analyses of the efficacy and safety of bevacizumab in mCRC have been published so far, but this is the first to include all data from 7 phase II or III randomized clinical trials of bevacizumab used in first- or second-line therapy. One of the advantages of this meta-analysis—which compared 1990 patients who received both bevacizumab and chemotherapy to 1773 patients who received chemotherapy alone—is that it permitted subgroup analyses for such factors as type of chemotherapy and KRAS mutation status. In fact, the overall analysis not only confirmed favourable reductions in the HR for OS (0.80; P=0.0003) and PFS (0.57; P<0.0001), but it also permitted relative benefits to be evaluated across subgroups. Bevacizumab may yield a greater OS benefit in combination with irinotecan (HR 0.71) than oxaliplatin (HR 0.87) and in KRAS wild-type tumours (HR 0.70) than KRAS mutant tumours (HR 0.85), but an overlap in the confidence intervals prevents definitive conclusions, as well as the similar effect in PFS for the KRAS status does (HR 0.57 and 0.54, respectively). The meta-analysis of safety reflected that seen in individual studies. While there was a slightly greater proportion of patients on bevacizumab with any adverse event of grade ≥3, the differences were largely limited to VEGF-related effects, such as hypertension (7.7% vs. 1.6%).
Questions and answers with Dr. Niall C. Tebbutt, Austin Health, Heidelberg, Victoria, Australia
Q: Were there any surprises in this meta-analysis relative to those previously published?
A: The data have been relatively consistent across individual studies and across meta-analyses. With the addition of bevacizumab to chemotherapy, outcomes in mCRC improve. This was seen across chemotherapy intensity, chemotherapy backbone and KRAS status. Not all the subgroups were large enough to demonstrate a statistically significant OS but the data all moved in the same direction and there was a statistically significant PFS advantage for all groups evaluated.
Q: Bevacizumab has been generally well tolerated, but the rate of grade ≥3 adverse events was greater in the group that received this agent. What were the most significant tolerability or safety issues?
A: The increased rate of adverse events is related to the effects of anti-VEGF, so this includes hypertension, rash and wound healing complications, but there is no significant increase in the types of adverse events that bother patients most, such as nausea and vomiting, neuralgia or neutropenia. Bevacizumab has a reputation for being well tolerated, particularly when compared to cytotoxic chemotherapy, and this is supported by the data from the meta-analysis.
Q: Do you feel that enough clinical experience has been collected with bevacizumab that clinicians should feel comfortable that the activity and safety of the drug has been well described?
A: In mCRC, we do have a relatively large data pool that has generated consistent information. It is important to continue to document the long-term safety of any drug, but there is now substantial evidence that bevacizumab is effective and safe in the treatment of mCRC.
ABSTRACT 3555
Phase II study of panitumumab (P) in combination with FOLFOXIRI as first-line treatment of metastatic colorectal cancer (mCRC): Activity in molecularly selected patients (pts).
S. Lonardi, L. Fornaro, F. Bergamo, M. Schirripa, G. Aprile, M. Morvillo, G. Masi, F. Loupakis, L. Calvetti, C. Cremolini, L. Salvatore, A. Zaniboni, V. Zagonel, A. Falcone
Background: GONO-FOLFOXIRI demonstrated higher activity and efficacy compared to FOLFIRI. P with oxaliplatin- or irinotecan-based doublets is feasible and associated with improved activity in KRAS codon 12-13 wild-type pts. BRAF and other RAS rare mutations have been suggested as additional potential biomarkers for anti-EGFR agents.
Methods: Pts with untreated unresectable mCRC and wild-type BRAF-RAS genes were enrolled in this GONO multicenter phase II trial of biweekly P 6 mg/kg d1 with a modified FOLFOXIRI regimen (IRI 150 mg/m2 d1, OXA 85 mg/m2 d1, l-LV 200 mg/m2 d1 and 5FU 3000 mg/m2 48-h continuous infusion d1, reduced to 2400 mg/m2 due to grade 3-4 toxicity in 2 of first 3 pts enrolled). Primary end-point was response rate (RR, RECIST Criteria). Based on a two stage Simon’s Minimax design (P0=60%, P1=80%; a=0.05, ß=0.2) at least 26 responses on 36 evaluable pts should be observed to satisfy the primary end-point.
Results: 37 out of 87 screened pts were enrolled (M/F, 57/43%; median age 63 years, range 33-72; ECOG PS 0/1-2, 76/24%; primary colon/rectum, 70/30%; primary on site, 42%; sites of disease single/ multiple, 54/46%; liver only mts, 35%). Among the first 3 pts treated with 5FU 3000 mg/m2, 2 experienced SAEs (1 grade 4 diarrhea and neutropenia; 1 grade 3 diarrhea). Grade 3-4 toxicities observed among the 34 pts treated at the amended dose were: neutropenia 53% (2 febrile neutropenia); diarrhea 32%; stomatitis 15%; neurotoxicity (grade 2-3) 30%; cutaneous rash 24%. Delays or dose reductions were needed only in 9% and 10% of the total 310 cycles, respectively. One SAE (febrile neutropenia and sepsis) resulting in pt death occurred after amendment. 32 partial responses, 4 disease stabilizations and 1 progression were observed, with a RR of 86% (95% CI: 75-97%). 9 pts underwent local procedures on metastases, achieving an R0 resection in 8 and a pathologic complete response in 3 of them. At a median follow-up of 7.4 months mPFS has not been reached.
Conclusions: In molecularly selected unresectable pts adding P to FOLFOXIRI resulted in high activity and interesting resection rate. The regimen appears feasible. Further follow-up is needed to assess long-term outcome.
Commentary on abstract 3555
Molecularly targeted therapy, including cetuximab, which inhibits EGFR, has been associated with improved outcomes in mCRC. In this phase II trial, panitumumab, a MAb that binds to EGFR, was evaluated in previously untreated mCRC patients with wild-type BRAF and RAS genes. The modified FOLFOXIRI regimen in this study was combined with a biweekly dose of panitumumab. This study documented a high rate of activity and toxicity with panitumumab and FOLFOXIRI. The median PFS had not been reached after 7 months of follow-up. Grade 3 or 4 toxicities were seen in 34 of the 37 patients enrolled, and 10% required a dose reduction. Of the 35 evaluable patients, there was one progressive disease observed on therapy and 32 patients achieved an objective response. The regimen was characterized as highly active. The authors were further encouraged by the fact that 8 of the patients were able to undergo R0 resections of metastases with a pathologic complete response achieved in 3 of them.
Questions and answers with Dr. Sara Lonardi, Veneto Institute of Oncology, IOV-IRCCS, Padua, Italy
Q: In a phase III trial published in late 2010, panitumumab was associated with a significant improvement in PFS when combined with FOLFIRI in second-line treatment of patients with mCRC and wild-type KRAS. What was different about this study?
A: Several years before that, our group [Gruppo Oncologico del Nord-Ovest] compared FOLFOXIRI to FOLFIRI as first-line treatment for mCRC and found the FOLFOXIRI regimen to provide both a significant OS and a PFS advantage. However, neither arm in this study received a targeted therapy. We wanted to evaluate the same regimen with panitumumab but decided to confine enrolment to patients with wild-type genes for BRAF and RAS because of evidence that these are biomarkers for increased susceptibility to this targeted agent.
Q: The response rates were high but the rates of adverse events also seemed substantial. Is this of concern?
A: We did amend the dosing regimen because of a high rate of serious adverse events, including one case of grade 4 diarrhea and 2 cases of febrile neutropenia. After the regimen was amended, there was still one case of febrile neutropenia and sepsis resulting in death, but we believe the adverse events are manageable given the high rate of activity.
Q: It is surprising that almost a third of evaluable patients underwent resection of metastases. Was this expected?
A: This is encouraging in a second-line treatment population. In our study comparing FOLFOXIRI to FOLFIRI first-line, we also associated FOLFOXIRI with a much higher rate of objective responses and a greater opportunity for resection of metastases, including a higher number of R0 resections. We even had an R0 resection in this most recent series with panitumumab. These data encourage a controlled trial to better evaluate the safety and efficacy of panitumumab.
ABSTRACT 6018
Use of palliative chemotherapy and targeted agents in elderly patients with metastatic colorectal cancer (mCRC).
M. Chan, D. J. Renouf, C. Speers, W. Y. Cheung
Background: Elderly patients are increasingly diagnosed with advanced cancers, but they are consistently underrepresented in clinical trials, which may lead to undertreatment. Our aims were to 1) evaluate the impact of advanced age on patterns of first-line chemotherapy and bevacizumab use in mCRC, 2) examine the reasons for treatment choices and 3) compare adverse events and treatment discontinuations in elderly vs young patients.
Methods: A random sample of mCRC patients diagnosed from 2006 to 2007 and referred to any 1 of 5 regional cancer centers in British Columbia, Canada was reviewed. Summary statistics were used to describe treatment patterns between the elderly (>/=70 years) and young (<70 years). Cox regression was used to determine the effect of systemic therapy on overall survival, controlling for age and confounders.
Results: We identified 800 patients: 43% elderly and 57% young; 56% men; and 26 / 36 / 38% ECOG 0 / 1 / 2+, respectively. Fewer elderly patients were given chemotherapy (52% vs 79%, P<0.001). Among those treated, most common first-line palliative regimens for elderly vs young included: capecitabine (50 vs 15%), FOLFIRI (26 vs 38%), and FOLFOX (15 vs 37%) (all P<0.001). Those aged >/=70 were also less likely to receive bevacizumab in their regimens (22 vs 50%, P<0.001). The most frequent reasons for no systemic therapy were similar between age groups: patient choice (31 vs 28%), poor ECOG (16 vs 17%), and significant co-morbidity (11 vs 13%). Risk of chemotherapy (P=0.30) and bevacizumab (P=0.39) adverse events were comparable between elderly and young as were rates of early chemotherapy (P=0.07) and bevacizumab (P=0.79) discontinuation. Receipt of systemic therapy +/- bevacizumab was associated with improved survival from mCRC (HR for death 0.50, 95% CI 0.31-0.62, P<0.001), regardless of advanced age (P interaction for age and treatment = 0.33).
Conclusions: Elderly patients with mCRC are more likely to receive no chemotherapy, capecitabine monotherapy, or a regimen without bevacizumab. However, in carefully selected elderly patients, adverse events, treatment discontinuations, and overall survival benefit from treatment appear similar to those observed for younger patients.
Commentary on abstract 6018
One of the most notable features of targeted molecular therapies has been their tolerability relative to cytotoxic agents. This favourable tolerability may prove to be particularly important if targeted therapies are employed sequentially in sustained maintenance regimens in order to slow or halt tumour growth. In patients with advanced cancer when cure is not the goal, the tolerability of molecular agents is particularly welcome because of the importance of extending survival with an acceptable quality of life. This goal would not be expected to be different in older than in younger patients, but the data from this study suggest that individuals with mCRC older than age 70 years are less likely than those younger to receive a targeted molecular therapy even though the relative benefit from targeted therapies in older and younger individuals appears to be similar. The reasons that older individuals do not receive targeted therapies may be complex, but these data raise the possibility that there is an age bias for prescribing these agents.
Questions and answers with Dr. Matthew Chan, British Columbia Cancer Agency, Vancouver
Q: Do these results suggest older people with mCRC are less likely to receive any therapy or just less likely to receive targeted therapy?
A: Patients over the age of 70 years were less likely to receive any therapy overall than younger patients, but our predominant finding was that the elderly were more likely to receive capecitabine monotherapy than younger patients, who were more likely to receive a combination like FOLFIRI or FOLFOX. However, older patients were also half as likely to receive bevacizumab (22% vs. 50%; P<0.001).
Q: Do you have an explanation for these patterns?
A: We did look at the reasons why patients did not receive a particular regimen and the reasons in both arms were fairly similar. The main reason was a personal decision that may or may not have been physician-influenced, but some records did cite comorbidities or poor ECOG status as a reason for not offering treatment. I think there is some concern among patients and physicians that the side effects of targeted therapy, such as rash and hypertension, are greater in the elderly, but the data do not support this.
Q: Do you feel that the elderly are being under-treated?
A: The elderly are often under-represented in clinical trials, so there may be some genuine confusion about the safety of bevacizumab and other targeted agents in elderly individuals. Perhaps some physicians think that adding targeted therapies is a more aggressive strategy that might be less warranted in older than younger patients, but treatment of mCRC is typically palliative. There is no reason to think that the survival benefit associated with targeted therapy would be less important in an elderly patient than in a younger individual when the risk of adverse events is similar.