Reports
A new option for the inoperable: Insights into management of NF1-associated plexiform neurofibromas
This report is based on medical evidence presented at sanctioned medical congress, from peer reviewed literature or opinion provided by a qualified healthcare practitioner. The consumption of the information contained within this report is intended for qualified Canadian healthcare practitioners only.
PRIORITY PRESS - NF Conference 2023: Children’s Tumor Foundation (CTF)
In-person/virtual, Scottsdale, Arizona / June 24–27, 2023
Scottsdale – The NF Conference is the flagship annual meeting of the US-based Children’s Tumor Foundation. This June, more than 700 participants from around the world gathered at the Fairmont Scottsdale Princess in Scottsdale, Arizona, to discuss the latest research, opinions, and clinical guidance about the rare hereditary cancer disorders neurofibromatosis and schwannomatosis. Neurofibromatosis type 1 (NF1) is the most common form of neurofibromatosis, caused by a mutation in the NF1 gene that codes for the tumor suppressor protein neurofibromin, resulting in dysregulation of the RAS-MAPK signalling pathway. The clinical presentation of NF1is highly variable but the most common manifestations include changes in skin pigmentation (most typically cafe´- au-lait macules and unusual axial and/or inguinal freckling), learning and developmental delays, and the development of multiple neurofibromas on the skin (cutaneous neurofibromas, cNF) and/or peripheral nerve sheaths (plexiform neurofibromas, PN). This report will focus on insights into the importance of PN in patients with NF1, and on advances in the management of NF1-associated PN.
Chief Medical Editor: Dr. Le´na Coi¨c, Montre´al, Quebec
“Plexiform neurofibromas can occur in up to 50% of patients who have NF1, and they can be painful and life-threatening,” said Theresa Dettling, Alexion Pharmaceuticals, in a podium presentation. Until recently, surgery was the main option for management of symptomatic PN; however, as Ms Dettling explained, “Many times surgical removal is challenging or can be impossible, due to the size of the PN and its location relative to vital structures.” In a chart review of pediatric patients with inoperable NF1-related PN in the era before targeted pharmacologic treatments were widely available, Ms Dettling and colleagues showed that disease progression occurred in about three-quarters of patients, generally involving increased pain and disfigurement. Inoperable PN was also associated with significant healthcare resource use; patients attended a mean of 6.1 outpatient visits per year and almost half of patients required hospitalization at some point during the 8.3-year study period. “There is a substantial burden associated with the monitoring and the treatment of patients with NF1-PN, as well as a considerable burden to the healthcare system,” she concluded.1
Selumetinib is an oral medication that inhibits MEK, a key kinase in the RAS-MAPK signalling cascade. In 2022 it became the first pharmacologic option approved in Canada for the management of symptomatic, inoperable NF1-PN, in patients aged 2 to 18 years. In the phase II SPRINT trial, about two-thirds of patients achieved an objective response (≥20% reduction in PN volume and/or disappearance of the target PN) to selumetinib. Across all trial participants and all levels of response, over 80% of patients reached a duration of response of at least a year.2
Insights into selumetinib for management of inoperable NF1-associated PN
Now that selumetinib is available in several countries for management of inoperable NF1-PN, new insights are emerging into its impact in real-world practice. Dr Thorsten Rosenbaum, Sana Kliniken, Duisburg, Germany, reviewed his centre’s experience using the MEK inhibitors trametinib (off-label, used up until 2021) and selumetinib (approved by the EMA in 2021) over the past 5 years in 24 patients with NF1- PN. Ten patients were treated with trametinib then switched to selumetinib when it became available, while the other 14 received selumetinib only. MRI volumetric measurements were available for 21 of the patients, and showed that 71.4% of patients (n=15) experienced tumour volume reduction with MEK inhibitor treatment, with an average reduction of 23% +/- 16.9% (range 2.7%–72.8%). The most common adverse events were rash, paronychia and nausea. “Treatment of NF1-associated PN with selumetinib leads to robust tumor volume reduction in the majority of patients,” concluded the authors. “Generally, therapy-related side effects are mild and manageable and appear to occur less frequently in young children.”3
Theresa Dettling and colleagues presented the results of a survey of 16 caregivers of pediatric patients with NF1- PN. “The aim of this study was to better understand the experience of children with NF1-PN from the perspective of their caregiver(s) following initiation of selumetinib treatment,” explained the authors. The key goals of selumetinib treatment included tumour reduction (43.8% of respondents) or stabilization (37.5%), and reduction of tumour-related pain (31.3%). Most caregivers reported either tumor stabilization or shrinkage since initiating selumetinib treatment. A majority of patients also experienced improvements in caregiver- reported pain and fatigue (Figure 1A) and social and emotional functioning (Figure 1B).4
Figure 1. Impact of selumetinib on caregiver-reported (A) pain and fatigue, and (B) social life, mental life, emotion, and learning
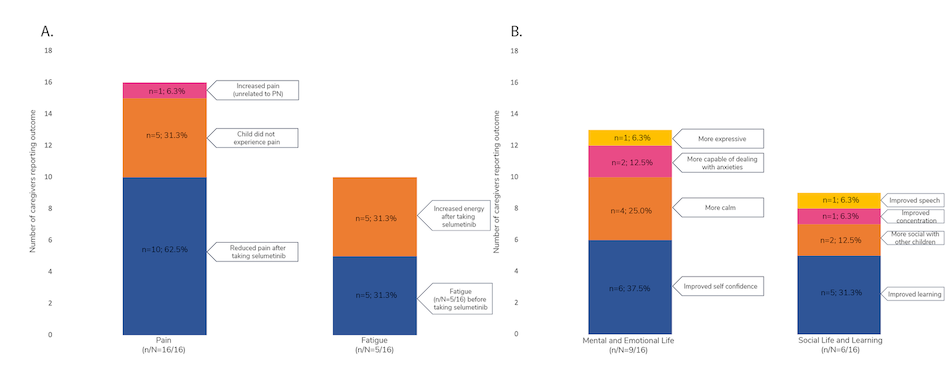
n, number of caregivers reporting improvements in the specified category; N, total number of caregivers included in the study; PN, plexiform neurofibroma. Adapted from Dettling T et al., poster 32 at NF Conference 2023.4
Potential future directions with MEK inhibitors
The current dosing instructions for selumetinib specify that it should be taken on an empty stomach, with no food intake in the two hours prior to dosing or for one hour afterward, based on prior pharmacokinetic (PK) studies showing a food effect on absorption.2 With twice-daily dosing, this can therefore have a significant impact on patient convenience and the timing of meals and medication doses. “That’s why we want to further understand the effect of food on selumetinib PK,” said Million Arefayene, Alexion Pharmaceuticals, to introduce his analysis of PK parameters for selumetinib and its active metabolite across participants in 15 clinical trials. The study included data from 511 subjects, including 91 children and 86 adults with NF1, and 334 healthy adult volunteers. Compared to the fasted state, exposure to selumetinib as measured by the area under the curve (AUC) was reduced slightly when the dose was taken with either a low-fat (22.5% reduction) or high-fat meal (20.8% reduction). However, these reductions were not deemed to be clinically significant, because within the dose range tested, small changes in selumetinib exposure have a minimal effect on therapeutic response. Similar results were seen for the active metabolite, suggesting that with further research, the dosing instructions in the approved selumetinib label could potentially change in future. “We are excited to share these findings with the scientific community, and we are encouraged by the potential to further advance care for the NF1 community and possibly to ease the treatment burden for the children and their families,” said Mr Arefayene. “We are evaluating the data and next steps and will share further information in due course, but I think it is important to emphasize at this point that selumetinib is currently approved for those in a fasted state.”5
Data were also presented for two additional MEK inhibitors, trametinib and binimetinib, in the management of NF1-PN. Neither of these medications is currently approved for NF1-PN in Canada.
Trametinib is a MEK inhibitor indicated for treatment of certain melanomas and lung cancers that has been used off- label in NF1-PN. Since the evidence base in NF1 and other neurological tumours is limited, the TRAM-01 phase II, open- label, non-randomized study was initiated by Dr Se´bastien Perreault, CHU Ste-Justine, Montreal, and colleagues, to investigate the safety and efficacy of trametinib in several types of neurological tumours including a cohort of patients with NF1-PN (n=46). Most adverse events were grade 1 or 2 and could be managed without dose modification, although 9 patients required dose reductions due to adverse events (mainly cutaneous) and 4 patients discontinued treatment due to AEs. Just under half of patients achieved a partial response (≥20% tumour reduction on volumetric analysis) during treatment, with a median time to response of 11 months. However, by the end of 18 months of treatment, some patients were losing their treatment response, with 2 patients experiencing progressive disease; a year after stopping treatment, only about one-third of patients still retained their partial response. “In conclusion, trametinib demonstrated efficacy for treatment of PN,” said Dr Perreault. “Treatment was overall well tolerated, but similar to what others have shown, it seems that prolonged treatment is probably needed for most patients.”6
Binimetinib is another MEK inhibitor currently indicated for management of melanoma but not of NF1-PN. Dr Alyssa Reddy, UCSF Benioff Children's Hospitals, San Francisco, and colleagues reported on a follow-up to a phase II trial of binimetinib in which patients who experienced PN progression after completing the initial 24-month study were eligible for binimetinib re-treatment. Although most patients in the primary trial achieved a partial response, after stopping binimetinib 78% of pediatric patients and 40% of adult patients experienced a recurrence of progressive disease. Most patients achieved a re- response to binimetinib once treatment was re-initiated. “These data suggest most pediatric patients require prolonged treatment to maintain a partial response,” concluded the authors. “Further studies are needed to determine optimal duration of therapy and continued monitoring for toxicity is warranted.”7
Conclusions
Plexiform neurofibromas can have a significant impact on the lives of patients with NF1 and on their interactions with the healthcare system. Selumetinib is a new treatment option for inoperable NF1-PN that may have benefits for clinical outcomes and the experiences of patients and their caregivers. Key areas for further research include the impact of food intake on selumetinib pharmacokinetics and the clinical efficacy and safety profiles of additional MEK inhibitor options.
References