Reports
More targets, more options, more awareness: Moving toward a personalized treatment approach in PNH
This report is based on medical evidence presented at sanctioned medical congress, from peer reviewed literature or opinion provided by a qualified healthcare practitioner. The consumption of the information contained within this report is intended for qualified Canadian healthcare practitioners only.
PRIORITY PRESS - EHA2023: European Hematology Association (EHA)
In-person/virtual, Frankfurt, Germany / June 8–11, 2023
Frankfurt – Paroxysmal nocturnal hemoglobinuria (PNH) is an acquired disorder of hematopoietic stem cells that results in the complement-mediated destruction of red blood cells (RBCs). Although PNH is a rare disease, interest and awareness are growing as we develop further insights into the mechanisms of disease and possible therapeutic targets. At the recent annual meeting of the European Hematology Association, five sponsored symposia, three podium presentations, and over 30 posters and abstracts explored the latest data on PNH. This report will focus on the latest clinical data for established and emerging treatment options, and how these may eventually help clinicians to provide more individualized management for each patient with PNH.
Chief Medical Editor: Dr. Le´na Coi¨c, Montre´al, Quebec
In PNH, RBCs lack the protective glycoproteins to defend them against complement-mediated destruction. RBCs are primarily destroyed through intravascular hemolysis (IVH), leading to hemolytic anemia and an elevated risk of thrombosis and bone marrow failure.
Eculizumab and ravulizumab are selective inhibitors of the terminal complement component C5 of terminal complement that have become established as the standard of care in Canada and other countries. Several presenters at EHA 2023 recognized the profound impact that these medications, particularly eculizumab, have had on PNH management. “Right from the first patient in May 2002, [patients treated] with C5 inhibition have been able to remain alive with thrombosis prevention and an improvement in survival,” said Dr Austin Kulasekararaj, King’s College Hospital, London, UK.1
“We lived through a revolution in 2005 when [eculizumab] became available – before that, patients were dying,” said Dr Re´gis Peffault de Latour, Saint-Louis Hospital, Paris, France. “Now [that survival has improved] we are entering a new era where we want to improve their quality of life.”2
Terminal Complement Inhibition
Standard-of-care C5 inhibitors: Eculizumab and ravulizumab
Eculizumab has been the standard of care for PNH in many countries since the early 2000s. More recently, its derivative ravulizumab has become widely available. Both are administered intravenously: every 2 weeks for eculizumab, every 8 weeks for ravulizumab.
In a poster presentation, Dr Kulasekararaj and colleagues compared the long-term outcomes with ravulizumab to those of eculizumab in a phase 3 study in eculizumab-experienced patients. During ravulizumab treatment, lactate dehydrogenase (LDH) levels, a key marker of IVH, remained under 1.5 times the upper limit of normal (Figure 1), similar to the findings with eculizumab. Overall survival of ravulizumab-treated patients over the 4-year follow-up was 98.4% and none of the deaths were related to treatment. The safety profile was consistent with shorter-term analyses. “These long-term results further support the use of ravulizumab as the first-line treatment of choice for patients with PNH, where available,” concluded the authors.3
Figure 1: Absolute mean change in LDH with long-term ravulizumab or eculizumab
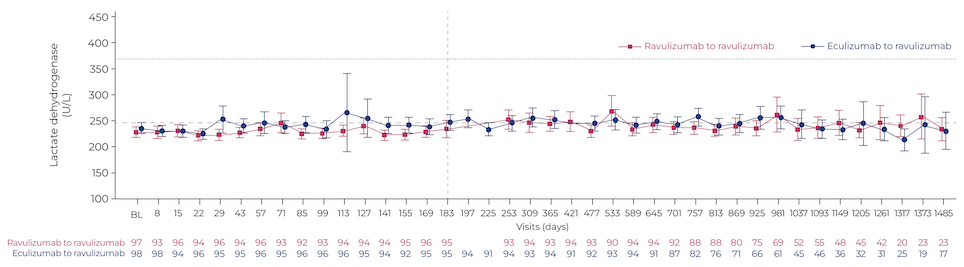
Adapted from Kulasekararaj et al., Poster P772, EHA 2023.3
Novel C5 inhibitor: Crovalimab
Given the established success of C5 blockade for reducing IVH and improving patient outcomes, additional C5 inhibitors are now in development. Two presentations at EHA 2023 outlined phase 3 trial data for the novel C5 inhibitor crovalimab (not currently approved in Canada), which is administered subcutaneously every 4 weeks.
Dr Kulasekararaj reported data from the randomized,open-label COMMODORE 1 trial of crovalimab versus eculizumab in eculizumab-treated patients. Patients who switched to crovalimab showed similar results to those who stayed on eculizumab for IVH control, transfusion avoidance, breakthrough hemolysis (BTH), and fatigue. The safety profile of crovalimab was generally similar to that of eculizumab, except that more than one-third of crovalimab-treated patients experienced a novel adverse event, type 3 hypersensitivity reactions driven by drug-target-drug complexes.4 Similar efficacy results for LDH control, transfusion avoidance, and BTH were seen in the COMMODORE 2 trial in treatment-nai¨ve patients, presented by Dr Alexander Ro¨th, University Hospital Essen, Germany.5
Proximal Complement Inhibitors
“When you're speaking about eculizumab, we all feel very safe, because we have 25 years of treatment. said Dr Peffault de Latour. “But some patients are still anemic and may need to be transfused.”2 One contributing factor to residual anemia could be extravascular hemolysis (EVH). “[EVH] has been identified as an iatrogenic effect in the context of C5 inhibitors, irrespective of the compound you’re using,” explained Dr Kulasekararaj. In brief, C5 blockade leads to accumulation and deposition of C3 on RBCs, leading to EVH.1 One potential strategy for controlling EVH is inhibition of proximal components of complement; new clinical trial data on several proximal inhibitors were presented at EHA 2023.
Factor B Inhibitor: Iptacopan
Iptacopan (not currently approved in Canada) is an orally administered inhibitor of factor B, a key driver of C3 production in the alternative complement pathway. The core results from a phase 3 randomized, open-label trial of iptacopan were reported at ASH 2022; over 24 weeks of treatment, most iptacopan-treated patients experienced an increase in hemoglobin from baseline of at least 2 g/ dL and an absolute hemoglobin level over 12 g/dL.6 An additional analysis, presented at EHA 2023 by Dr Antonio Risitano, University of Naples, Italy, showed that iptacopan monotherapy led to an increase in the size of the PNH RBC clone to over 90% of the total RBC population. Dr Risitano pointed out that in contrast to other hematologic conditions (e.g., malignancies) where the goal is a decrease in the clone of diseased cells, in PNH this is a desirable effect as it indicates that hemolysis is being effectively controlled, allowing for the survival of PNH RBCs. However, this population may be vulnerable to BTH if C3 inhibition is withdrawn (e.g., missed doses) or in situations of high complement activation (e.g., infection). “This may raise the debate about whether [iptacopan] monotherapy will be better or [whether we should look at] combination treatments,” said Dr Risitano.7
Factor D inhibitor as add-on therapy: Danicopan
Danicopan (not currently approved in Canada) is an orally administered inhibitor of factor D, another key factor for C3 production. In an e-poster, Dr Jong Wook Lee, Seoul St. Mary’s Hospital, South Korea, and colleagues reported results from a phase 3 placebo-controlled trial of add-on danicopan in patients experiencing clinically significant EVH while on eculizumab or ravulizumab. After 12 weeks, patients on add-on danicopan experienced a mean hemoglobin increase of 2.94 g/dL, compared with 0.496 g/dL for patients maintained on eculizumab or ravulizumab monotherapy. Add-on danicopan also provided a higher rate of transfusion avoidance through week 12 (83.3% vs 38.1% for placebo). The authors concluded that, “Danicopan add-on to eculizumab or ravulizumab significantly improves hemoglobin and reduces the need for transfusion by addressing clinically significant EVH while maintaining essential control of terminal complement activity and IVH.”8.
Perspectives for Canadian Clinicians from International Experts
Overall, the presenters at EHA 2023 felt optimistic that our expanding understanding of PNH pathophysiology and new and emerging treatment options will give clinicians the opportunity to enhance patient care. “If [novel treatment options] all get through the approval process and we have options available for patients, I think it's fantastic,” said Dr Morag Griffin, St. James University Teaching Hospital, Leeds, UK. “Not everybody wants a tablet, not everyone wants a subcutaneous [drug], not everybody wants an IV. So to be able to offer your patients a variety of options, I think is really important.”9
“I think C5 [inhibition] still has a significant role to play in the treatment of PNH. It manages the life-threatening immediate IVH risk,” said Dr Griffin. “Whilst the majority of patients will develop EVH on a C5 inhibitor, that doesn't necessarily need treating.”9 When asked how to manage clinically significant EVH, Dr Kulasekararaj said, “The first thing I would do is have a discussion with the patient to see how much it has impacted on their quality of life. [I would also discuss their disease] stability – because remember, these patients have been on the drug for many years, and have confidence in their long-term treatment, and [they also appreciate] the convenience with eight-weekly ravulizumab.” He also discussed the option of switching to pegcetacoplan (where approved), or of enrolling in a clinical trial of a novel agent.1
“We have to be careful – PNH is the most devastating thrombotic state and you have the feeling that we are forgetting that [in the trial endpoints of novel therapies],” cautioned Dr Peffault de Latour. “Please don’t forget that PNH is a predisposition to thromboses that are of bad prognostic significance, even in 2023.”2
Conclusions
As our understanding of PNH evolves and novel treatment options become available, clinicians will be better able to offer individualized care that recognizes each patient’s clinical and personal goals. C5 inhibition remains the standard of care and new data show that ravulizumab has similar long-term outcomes to eculizumab. Proximal complement inhibition may hold promise for addressing EVH but long-term data are needed to understand their impact on key clinical outcomes (e.g., thrombosis) and the risk of BTH.
References
1. Lee JW, et al. Towards Better Characterization of Haemolysis in Paroxysmal Nocturnal Haemoglobinuria (PNH). Satellite symposium at EHA 2023, June 9, 2023.
2. Schrezenmeier H, et al. Looking from the past to the future for patients with paroxysmal nocturnal hemoglobinuria (PNH): An exploration of novel treatment approaches. Satellite symposium at EHA 2023, June 8, 2023.
3. Kulasekararaj A, et al. Poster P772 at EHA 2023, June 8–11, 2023.
4. Kulasekararaj A, et al. Oral presentation S183 at EHA 2023, June 8–11 2023.
5. Ro¨th A, et al. Oral presentation S181 at EHA 2023, June 8–11, 2023.
6. Peffault de Latour R, et al. Late-breaking oral presentation LBA-2 at ASH, Dec 10–13, 2022.
7. Risitano AM, et al. Oral presentation S182 at EHA 2023, June 8–11, 2023.
8. Lee JW, et al. Poster P771 at EHA 2023, June 8–11, 2023.
9. Griffin M, et al. Redefining treatment goals in Paroxysmal Nocturnal Haemoglobinuria (PNH): Reaching new heights in patients with ongoing anaemia. Satellite symposium at EHA 2023, June 10, 2023.