Reports
Closing the Gap on N. meningitidis: Potential for Imminent Disease Control
This report is based on medical evidence presented at sanctioned medical congress, from peer reviewed literature or opinion provided by a qualified healthcare practitioner. The consumption of the information contained within this report is intended for qualified Canadian healthcare practitioners only.
PHYSICIAN PERSPECTIVE - Viewpoint based on the following articles: Santolaya et al. Lancet 2012;379:617-24, Gossger et al. JAMA 2012;307(6):573-82.
April 2012
Guest Editor:
Ronald Gold, MD, MPH
Former Head, Division of Infectious Disease
The Hospital for Sick Children
Toronto, Ontario
Professor of Pediatrics (Retired)
Faculty of Medicine, University of Toronto
Medical Advisor
Meningitis Research Foundation of Canada
Introduction
The epidemiology of invasive meningococcal disease (IMD) in Canada changes over time and varies depending on geographic location, season and patient age. On average, 235 cases are reported annually. Of the 5 serogroups that cause IMD, only serogroup B disease has been uncontainable due to immunogenicity issues relating to the polysaccharide coat that defines each serogroup. Novel technology has now overcome the inherent challenges of developing a vaccine against serogroup B disease. The new multi-component serogroup B vaccine (4CMenB) has undergone extensive testing in infants and adolescents. It was shown that almost 100% of infants developed protective antibodies against 2 of the antigens and the majority against the other 2 antigens when given with and without routine childhood vaccinations. Responses from a pivotal adolescent trial similarly indicate that 2 doses of the vaccine, given 1 to 6 months apart, provide highly effective protection against meningococcal B infection in this age group as well. Several important unknowns still need to be answered concerning this novel vaccine but its anticipated availability will make control of IMD against all 5 serogroups potentially possible, especially in the most vulnerable age groups.
According to a 2009 statement from the National Advisory Committee on Immunization (NACI) (CCDR 2009;36:ACS-3), over half of all invasive meningococcal disease (IMD) in Canada reported in 2006 (the latest year for which complete data are available) was caused by serogroup B (i.e. 113 cases out of a total of 210 for that year); the highest attack rate was reported in infants <1 year of age, where 82% of IMD cases were caused by serogroup B. Serogroup B disease steadily declines until adolescence, at which point it spikes quite dramatically in adolescents and young adults between 15 and 24 years of age. In comparison, serogroup C disease caused only 43 cases in 2006; serogroup Y, 27 cases; serogroup W-135, 6 cases; and serogroup A, 2 cases. Other serogroups accounted for 19 cases out of the total reported for 2006. Nationally, prevention of serogroup B IMD is of imminent importance, especially in young infants and adolescents, the 2 most vulnerable age groups for serogroup B infection.
As NACI pointed out, the incidence of serogroup C disease has declined dramatically in recent years, likely due at least in part to the introduction of the meningococcal C conjugate vaccine in routine and catch-up programs in all provinces and territories. Rates of serogroup Y disease have remained stable over time while disease caused by both serogroup W-135 and A are rare in Canada.
The Quebec Experience
Currently the epidemiology in Canada indicates that serogroup B Neisseria meningitidis is now the number one cause of IMD across the country, where it causes between 100 and 300 cases of IMD each year. This is borne out by more recent epidemiology of IMD in Quebec. As reported by Dr. Rodica Gilca, Institut national de santé publique du Québec, Québec City, at the 2011 ESPID meeting, 97% of all IMD cases in individuals between 0 and 19 years of age in the last 3 years were caused by serogroup B, as was 97% of IMD in children under the age of 5.
Until about 2003 to 2004, serogroup B IMD was also uncommon in adolescents living in Quebec. Since then, the province has seen a very rapid increase in IMD due to serogroup B, its incidence increasing sixfold in adolescents between 15 and 19 years of age compared to previous periods. Of interest, this same increase has not been seen in children under the age of 5, among whom the incidence of serogroup B disease has remained relatively constant over the years in Quebec.
Prevention of Serogroup B Disease
Using the same process by which conjugate vaccines have been developed against the other 4 N. meningitidis serogroups, the development of a vaccine against serogroup B infection was not possible because its polysaccharide capsule too closely resembles neural cell adhesion molecules in humans, rendering candidate vaccines poorly immunogenic and possibly autoimmunogenic. In research that dates back over a decade, vaccine researcher Rino Rappuoli, PhD, Siena, Italy, eventually identified 3 novel antigens critical to the survival, fitness and virulence of the organism using a technology called “reverse technology.” (It is noteworthy that serogroups other than serogroup B also carry the same antigens.)
These antigens, now contained in the novel 4CMenB vaccine, include factor H-binding protein (fHbp); Neisserial adhesin A (NadA); and Neisseria heparin-binding antigen (NHBA). A fourth component is a serogroup B PorA-containing outer membrane vesicle previously used to control an outbreak of serogroup B disease in New Zealand (NZ), a known hyperendemic strain. (Serogroup B vaccines have been used successfully to combat epidemic outbreaks but the outer membranes used in these epidemic vaccines limit their effectiveness to the clonal outbreak strains.)
Small studies had previously demonstrated that the 4CMenB vaccine was immunogenic against reference strains in infants. More recently, a phase IIb, open-label, parallel-group, randomized, controlled trial by Gossger et al. (JAMA 2012;307(6):573-82) was carried out. Healthy full-term, 2-month-old infants were given 3 primary 4CMenB schedules: one at ages 2, 4 and 6 months together with routine infant vaccine (concomitant schedule); a second in which infants were given the 4CMenB vaccine at the same time points but routine vaccines were given separately at 3, 5 and 7 months (intercalated schedule); a third group in which infants received both the 4CMenB and routine infant vaccines at 2, 3 and 4 months (accelerated schedule); and a fourth control group who received routine infant vaccines only at 2, 3 and 4 months.
A total of 1636 infants were included in the modified intent-to-treat (ITT) immunogenicity analysis and 1599 in the per-protocol immunogenicity analysis. As investigators reported, after immunization with the 4CMenB vaccine plus routine infant vaccines given at either 2, 4 and 6 months or at 2, 3 and 4 months, 99% or more of infants developed human complement serum bactericidal activity (hSBA) titres of 5 or greater (1:5) against 2 vaccine antigens. Based on the ITT analysis, 79% of infants following the concomitant schedule also developed similar protective hSBA titres against a third vaccine antigen, as did 86.1% of infants following the intercalated schedule and 81.7 % of infants following the accelerated schedule (Figure 1.)
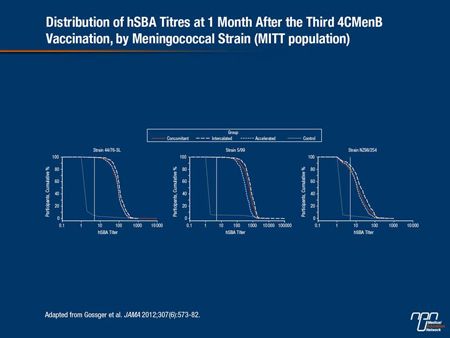
At the time of analysis, no strain specific for the NHBA antigen was available for use in the human complement bactericidal assay; antibodies to NHBA were thus measured by ELISA and expressed as geometric mean concentrations (GMCs). As investigators reported, GMCs against NHBA 1 month after the third dose of the 4CMenB vaccine were higher when it was given separately from routine infant vaccines than when it was given concomitantly with other vaccines.
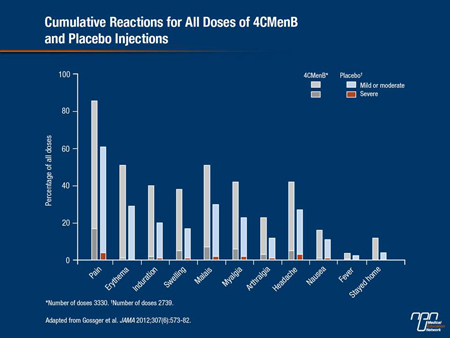
Adverse reactions were more common when the 4CMenB vaccine was given with routine infant vaccines than for controls who did not receive the meningococcal B vaccine. Specifically, 12 to 16% of infants who received the 4CMenB vaccine either in the concomitant or accelerated schedule experienced severe local pain vs. 1 to 3% of infants in the control group. The incidence of fever (≥38°C) was also reported in most infants in both the concomitant and the accelerated groups (76 to 80%) compared to about 50% of control infants. Rates of fever for infants in the intercalated group were also relatively high at about 70%, but it suggests that the 4CMenB vaccine appears to be less reactogenic when given separately rather than together with routine vaccines.
Fever
The occurrence of fever in young infants receiving the 4CMenB vaccine has been discussed as a possible deterrent for parents from having their infant vaccinated against serogroup B disease. But as physicians are already aware, fever occurs with almost every vaccine given to infants and it is more likely to occur in infants than in older children. Moreover, when parents involved in one of the infant studies were alerted to the possibility of fever, there was no difference in the number of visits made by parents to physicians because of fever. The occurrence of significant fever (≥40°C) has also been very uncommon in any of the infant studies.
Results from this study therefore indicate that the new 4CMenB vaccine is immunogenic against reference strains when given together with routine vaccines at either 2, 4 and 6 months of age or at 2, 3 and 4 months of age, nor does it interfere with responses to routine infant vaccinations. Findings also indicate that there can be a certain flexibility in the scheduling of the 4CMenB vaccine based on different infant immunization schedules used in different countries.
Adolescent Study
The same novel meningococcal B vaccine was again tested in a phase IIb/III study in 1631 adolescents between 11 and 17 years of age. As reported by Santolaya et al. (Lancet 2012;379:617-24), participants were randomized to receive either 1 dose; 2 doses given 1 month apart; 2 doses given 2 months apart; or 3 doses of the 4CMenB vaccine or 3 doses of placebo. (In Chile, where the study took place, 61% of IMD is caused by serogroup B.) At 6 months, those who had previously received either 1 or 2 doses of the 4CMenB vaccine in the primary phase were further randomized to receive the same vaccine or placebo; those receiving only placebo during the primary phase of the study received 1 dose of the 4CMenB vaccine. Adolescents who received 3 doses of the vaccine during the primary phase received placebo at month 6. Sera collected at baseline and 1 month after each injection was assessed by analyzing hSBA titres against 3 serogroup B reference strains.
The primary immunogenicity end point was the percentage of participants who achieved an hSBA titre of 4 or more, a level that has shown to be protective against IMD. After 1 dose of the 4CMenB vaccine, 93% of recipients achieved hSBA titres of 4 or more for the strain specific to fHbp. Ninety-six per cent of recipients achieved the same protective hSBA titres against NadA as did 93% to NZ outer membrane vesicles. After a second dose, over 99% of recipients had hSBA titres of 4 or more against the same 3 vaccine antigens and the effect was significantly superior for all antigens compared with a single dose of the same vaccine (P<0.0001). In contrast, a third dose of the vaccine did not increase the proportion of participants achieving protective hSBA titres.
As the authors pointed out, all antibody titres waned some 2 months after the first dose but levels remained protective in approximately 70% of recipients. Importantly, a booster response to a second or third dose of the vaccine at 6 months re-established protective antibody titres of 4 or more in 99 to 100% of recipients.
As was the case in the infant study, no suitable candidate strain was available to assess bactericidal responses against NHBA, 1 of the core components of the vaccine. Sera samples from each study group were therefore again tested by ELISA for antibodies to this antigen at each time point. ELISA results demonstrated the same pattern of antibody responses as hSBA titres did against other vaccine antigens, with a similar booster effect with a second dose. Most adverse events were injection-site reactions including, most commonly, pain; most were reportedly mild to moderate in severity, resolving within a few days of each vaccination. The most common systemic reactions were malaise, headache and fever ≥38°C but fever was not reported in more than 4% of vaccine receipts. The authors concluded that tolerability outcomes were acceptable (Figure 2.)
Potential Limitations
As editorialists Dr. Amanda Cohn and Dr. Nancy Messonnier, Centers for Disease Control and Prevention, Atlanta, Georgia, noted, meningococcal disease-causing strains vary by country. Surveillance studies suggest that the 4CMenB vaccine could be protective against approximately 75% of disease-causing strains now circulating in Europe and tests do not show major differences in B strains between Europe and the US. In Canada, the Canadian National Microbiology Laboratory is in the process of testing large numbers of group B strains from cases of B disease to establish the epidemiology of serogroup B disease here.
Concerns have also been raised about whether the 4CMenB vaccine will reduce nasopharyngeal carriage and produce herd immunity, as was seen following the introduction of the serogroup C conjugate vaccine in the UK and elsewhere. This is one of the current unknowns but studies are in progress to determine how the vaccine affects carriage. It is hoped that it will confer protection against B disease both in the individual vaccinees as well as others because of reduced carriage and spread of the bacteria.
Another unknown is the duration of protection. As the same editorialists mentioned, this may depend on the characteristics of the strains circulating in a country. However, studies do show that booster doses of the 4CMenB vaccine do give rise to protective antibody titres. Again, studies are ongoing to determine the optimal timing of a booster dose to maintain long-lasting protection. The 3 major proteins contained in the 4CMenB vaccine are not unique to serogroup B strains; rather, they occur in varying amounts in all serogroups causing IMD. Studies in adults have shown that vaccination with the 4CMenB vaccine produces bactericidal levels of antibodies not only against B strains but against strains from serogroups A, C and Y as well. Further studies are underway to determine how effective the vaccine might be against all disease-causing serogroups of IMD.
Summary
Historically, infants and adolescents, the most vulnerable groups for serogroup B IMD, remained at risk as a vaccine against serogroup B disease could not be developed that would provoke a protective response. Novel antigens involved in the survival, fitness and virulence of the organism have now been incorporated into a multicomponent serogroup B vaccine. Studies have shown the 4CMenB vaccine is safe, immunogenic and reasonably well tolerated in both infants and adolescents. This new vaccine, along with currently available quadrivalent meningococcal vaccines, will soon make it possible to prevent all IMD caused by any of the 5 main serogroups of N. meningitidis.