Reports
Early Intervention and a Tailored Approach: Opportunity for Improved Disease Control in the Management of Relapsing-Remitting Multiple Sclerosis
This report is based on medical evidence presented at sanctioned medical congress, from peer reviewed literature or opinion provided by a qualified healthcare practitioner. The consumption of the information contained within this report is intended for qualified Canadian healthcare practitioners only.
MEDICAL FRONTIERS - XXI World Congress of Neurology
Vienna, Austria / September 21-26, 2013
Vienna - Of all patients diagnosed with multiple sclerosis (MS), 90% will have relapsing-remitting multiple sclerosis (RRMS), the other 10% having a primary progressive form of the disease. Without treatment, approximately half of patients with RRMS will convert to progressive disease within a decade. Effective, well-tolerated treatments for RRMS thus have the ability to delay and potentially prevent the progressive phase of the disease and stabilize disability progression for many decades to come. Optimal clinical outcomes are best achieved with early intervention when accumulation of deficits is at a minimum. A plethora of new agents targeting RRMS is emerging from which physicians will need to choose the most suitable drug possible based on individual patient needs and disease burden.
Until recently, first-line treatment for RRMS consisted of some form of beta interferon (IFN b) or glatiramer acetate (GA). Both disease-modifying agents must be injected and side effects include not only injection-site reactions but influenza-like symptoms that can persist for several days after injection. Recently, researchers have characterized multiple molecular pathways giving rise to MS; by targeting specific points along these pathways, they have given birth to a plethora of new treatments that can stabilize disease and significantly improve long-term outcomes for RRMS patients.
The key to long-term stabilization is early intervention. As discussed by Dr. Mark Freedman, Professor of Neurology, University of Ottawa, Ontario, early demyelinating events occur well before patients present with the first clinical manifestation of MS. “When patients first declare their disease, there is already axonal damage,” he said. Physicians therefore need to determine where within the “window of opportunity” a patient might be to match treatment to underlying disease burden. If the risk profile suggests patients are at low risk for disease progression, “it is reasonable to start with an efficacious, low-risk therapy,” Dr. Freedman said. “Safety is first.”
On the other hand, if the risk profile suggests a more advanced stage of disease, “patients need more rapid and definitive control,” he added. “Efficacy comes first.” Response to treatment also needs to be monitored over the course of the first year; if response is not optimal, “you have to switch while they are still in that window of opportunity,” Dr. Freedman said. Patients may be switched back to a lower-risk treatment if response to more aggressive disease control proves optimal, he added.
If not, more aggressive therapy will need to be permanent, regardless of its risk. A number of years ago, the Canadian Multiple Sclerosis Working Group developed practical recommendations to help neurologists determine patient response to disease-modifying therapies and decide when they need to act. Recommendations are based on the frequency and severity of relapse; the length and extent of recovery following treatment; progression according to changes in the Expanded Disability Status Scale (EDSS), as well as changes from a previous MRI (Can J Neurol Sci 2004; 31:157-68).
“Not all patients are the same,” Dr. Freedman emphasized. “You have to characterize what is going on with individual patients, consider under which circumstances you need a more aggressive approach and consider what you must do if response is suboptimal.”
Burgeoning Options
With a burgeoning number of therapeutic options for RRMS, physicians also need to tailor drug treatment to individual patient needs, taking both efficacy and safety issues into account. One new oral agent recently approved by Health Canada for first-line treatment in RRMS is dimethyl fumarate (DMF) delayed-release capsules. In pivotal, Phase III randomized trials, DMF was found to be highly active in RRMS. Both DEFINE (Determination of the Efficacy and Safety of Oral Fumarate in Relapsing-Remitting MS) and CONFIRM (Comparator and an Oral Fumarate in RRMS) were similarly designed with patients randomized to the new oral agent at a dose of 240 mg, given either twice or 3 times a day, or to placebo. CONFIRM also included GA as a comparator.
In both trials, patients had an EDSS score of between 0 to 5.0 and ≥1 relapse in the 12 months prior to randomization or ≥1 gadolinium-enhancing (Gd+) lesion on brain MRI within 6 weeks prior to randomization. The primary end point of DEFINE was the proportion of patients who had relapsed by the end of 2 years while in CONFIRM, it was an annualized relapse rate. As noted by Dr. Thomas Berger, Head of Neuroimmunology and the MS Clinic, Innsbruck Medical University, Austria, the primary end point in DEFINE was a secondary end point in CONFIRM and vice-versa.
“This allowed investigators to compare results from both studies together in an integrated analysis,” he added. At the end of 2 years, the annualized relapse rate was approximately half that in both DMF treatment arms and in both trials compared with placebo controls (Table 1).
Table 1.
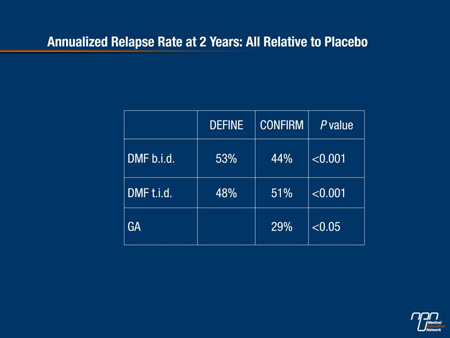
“As a surrogate mirror of the annualized relapse rate, there was also a highly significant and very substantial reduction in gadolinium enhancement (Gd+) in favour of the treatment arms vs placebo as well as in new or newly enlarging T2-hyperintense lesions at 2 years,” Dr. Berger added (Table 2).
Table 2.
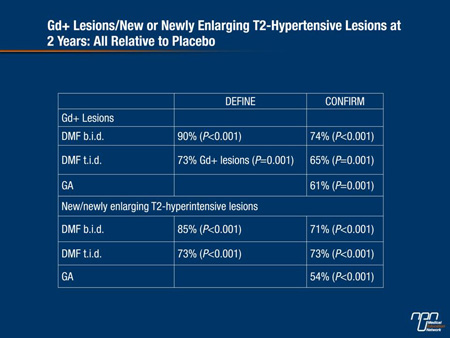
In a related poster by Fox et al. (Abstract 417) DMF treatment also reduced brain atrophy at the end of year 2 compared with either baseline or week 24 findings. The reduction was statistically significant for the twice-daily group relative to placebo (P<0.01). Researchers postulate that treatment reduces edema in the myelin sheath and helps preserve its integrity over time.
Importantly as well, both doses of DMF in both DEFINE and CONFIRM significantly slowed progression of disability. In DEFINE, the risk of disability progression was reduced by at least one-third over that of placebo controls, as Dr. Berger noted.
Nevertheless, the effect of both doses on sustained disability progression at week 12 was roughly the same relative to placebo in the integrated analysis of the 2 trials involving almost 2700 patients, as he also noted. Flushing and gastrointestinal (GI) events do occur significantly more often at both doses of DMF compared with placebo.
However, events taper off after the first month of treatment. “If patients are informed that these events may occur, they usually will make it through,” Dr. Berger said. As of March, 2013, no new or worsening safety signals have been identified in patients who continued to be exposed to DMF, some for up to 4 years, according to findings from ENDORSE, an extension trial of DEFINE and CONFIRM.
Lastly and importantly, there have been no reports of an increased risk of fetal abnormalities or adverse pregnancy outcomes with DMF exposure during the first trimester. Exposure was documented in 56 pregnancies in clinical trials to date. “We have many more females than males with MS,” Dr. Ralf Gold, Professor of Neurology, Ruhr-Universitat Bochum, Bochum, Germany, said in an interview. “And with females, you have maternity issues and the desire to become pregnant so it’s very important that we have drugs that do not appear to have any effects on pregnancy or the newborn.”
Highly Active RRMS
Natalizumab represents another valid disease-modifying option for the treatment of highly active RRMS despite an adequate course of either IFN b or GA. As described by Dr. Patrick Vermersch, Head of Neurology, University of Lille, France, the pivotal AFFIRM trial (N Engl J Med 2006;354:899-910) during which natalizumab was given by intravenous infusion every 4 weeks for over 2 years showed the selective adhesion-molecule inhibitor was highly effective against RRMS. At study end point, there was a 68% reduction in the relapse rate and a 42% reduction in sustained disability progression among patients randomized to natalizumab compared with placebo (P<0.001 for both end points). There was also a 92% reduction in Gd+ lesions and an 83% reduction in T2-hyperintense lesions in favour of the active therapy group.
Significantly more patients treated with natalizumab had both a sustained improvement in function and were free of disease activity compared with placebo at the same 2-year follow-up. The major concern with the use of natalizumab is the possibility that patients testing positive for the John Cunningham virus (JCV) could be at risk for progressive multifocal leukoencephalopathy (PML). Along with being anti-JCV antibody positive, risk factors for PML include exposure to the drug beyond 2 years and prior treatment with an immunosuppressant.
However, for anti-JCV negative patients, “there is virtually no risk of PML,” Dr. Vermersch emphasized. Even for patients who are anti-JCV positive, the risk of developing PML within the first 2 years of treatment is still low at less than 1/1000 patients, provided they have received no prior immunosuppressant therapy. “We have new expectations for the treatment of MS,” Dr. Vermersch said. “Not only do we expect to reduce the relapse rate and the risk of disability progression but we have now adopted the concept of ‘free of disease and MRI activity’. And with some potent drugs like natalizumab, it is possible to achieve this goal in a significant proportion of patients.”
Another potential for early active RRMS is alemtuzumab (Abstract 202). Alemtuzumab is given as 5 infusions initially and an additional 3 infusions are given 12 months later. While shown to be superior to IFN b-1a over 2 years (Lancet 2012; 380:1819-28), neurologists should be aware that the majority of patients experience injection adverse reactions (IARs) with alemtuzumab, though they are manageable, as noted by Dr. Jan Lycke, Associate Professor of Neurology, University of Gothenburg, Gothenburg, Sweden.
“There is also an increase in herpes infection,” he added—again manageable with administration of prophylactic acyclovir, 200 mg b.i.d., started immediately after the first infusion and continued for one month. The incidence of thyroid adverse reactions actually increased from year 1 to year 2 in the pivotal alemtuzumab RRMS trials, as Dr. Lycke observed, so physicians need to be aware that thyroid abnormalities do occur with treatment and do not necessarily resolve over time.
In contrast, the additional treatment course in the second year of the alemtuzumab clinical trials did not confer a cumulative risk of infection. For RRMS patients with visual dysfunction, alemtuzumab may also help improve visual acuity. In a study by Balcer et al., multicentre investigators randomized a total of 581 patients to either alemtuzumab, 12 mg/day IV on 5 consecutive days initially and 3 consecutive days 12 months later or to IFN b-1a, 44 μg given subcutaneously 3 times a week. MS functional composite scores along with binocular Sloan assessments were done twice a year and scores were combined to create a normalized 4-dimensional composite that incorporated visual impairment in the overall disability assessment.
Investigators observed that mean changes in low-contrast Sloan scores from baseline were smaller in patients who had received alemtuzumab compared with IFN b-1a and that differences between the 2 treatment arm were significant at 12 months (P=0.0019) although not at 24 months. They also noted that when low-contrast Sloan scores were added to MS functional composite scores, treatment differences again significantly favoured alemtuzumab at both 12 months (P=0.0001) and 24 months (P=0.029) and that fewer patients at 15.3% had worsening low-contrast Sloan scores at 12 months compared to those in the interferon arm at 23.6%.
Again at 24 months, results favoured alemtuzumab in terms of the proportion of patients who had worsened or remained stable although not significantly so. Investigators observed that the use of a visual component measure could enhance clinicians’ ability to detect treatment effects across a variety of clinical dimensions in MS trials.
pegIFN b-1a
There is no question that IFN b therapies reduce the annualized relapse rate and disability progression in patients with RRMS. IFN b therapies also delay progression to secondary progressive MS compared with placebo. On the other hand, IFN b therapies usually require frequent injections to optimize clinical outcomes, making adherence challenging. As discussed by Dr. Bernd Kieseier, Vice-Chair and Head, MS Outpatient Clinic, Heinrich-Heine University, Dusseldorf, Germany, pegylated IFN b-1a may provide equal therapeutic benefits to standard treatment but with less frequent dosing.
At the end of the first year of the 2-year ADVANCE trial, both biweekly and monthly pegIFN b-1a, 125 μg SC, reduced the annualized relapse rate compared with placebo, with a 36% reduction in relapse risk for the biweekly schedule and 28% reduction in relapse risk for the monthly schedule. The biweekly—though not monthly—schedule also led to a statistically significant 86% reduction in the mean number of Gd+ lesions at one year compared with placebo and a 67% reduction in new and newly enlarging T2 lesions again at 1 year.
“One year follow-up is too short to identify patients with neutralizing antibodies, but overall, there was no real signal of this happening at one year,” Dr. Kieseier said. “And the safety of pegIFN b-1a given SC reflects pretty much what we’ve learned to live with for established IFN b therapies.”
Summary
It’s been almost 20 years since the medical community had access to the first agents that could modify the course of disease in RRMS patients. Since then, researchers have illuminated the immunopathological changes that occur as the disease evolves and have learned about the constellation of risk factors that govern the accrual of permanent disability. Given that the majority of patients transition to the progressive stage of the disease, efforts have shifted towards providing effective interventions at a time when important deficits have not accumulated. Early initiation of effective, well tolerated and easy-to-administer oral agents such as DMF thus have the potential to buffer patients against disease progression and preserve physical, mental and social function and thus overall quality of life for years to come.
Questions and Answers
Questions and answers with Dr. Ralf Gold, Professor of Neurology, Ruhr-Universitat Bochum, Bochum, Germany.
Q: Were you surprised by how active DMF is in RRMS?
A: Not really, because I know the compound from a decade of research when we worked and studied it in the laboratory. But because the Phase IIb study by Kappos et al. found DMF had only IFN-like activity on relapse—in the range of 30 to 35%–some were surprised by the Phase III findings. People need to know, though, that it takes between 2 to 3 months before you reach the full activity of the drug and in a 6-month trial, you can’t get full activity.
Q: Are you finding DMF effects are consistent with longer-term exposure to treatment?
A: Results are still consistent out to 4 years. There is no indication that the therapeutic activity of DMF waxes and wanes; it remains constant. And patients who were in the placebo group originally [in DEFINE and CONFIRM] and then who were switched to DMF after 2 years, either 2 or 3 times a day, all had a clear and well-maintained benefit from the therapy with no red flags concerning side effects occurring in almost 10,000 patient-years.
Q: What do you see as the ideal niche for DMF in RRMS?
A: I do not see DMF as a niche. The drug led to a relapse reduction of about 50% without any long-term side effects so while we still need to do some basic blood work to obtain a complete blood count (CBC) at baseline, repeat it after 6 months of treatment and thereafter as clinically indicated, there’s not much more to it. So I think this drug can address the needs of a broad majority of RRMS patients. This is my hope because it can make MS patients stable very early on.