Reports
Examining New Treatment Options for Overactive Bladder Syndrome
This report is based on medical evidence presented at sanctioned medical congress, from peer reviewed literature or opinion provided by a qualified healthcare practitioner. The consumption of the information contained within this report is intended for qualified Canadian healthcare practitioners only.
PRIORITY PRESS - 107th Annual Meeting of the American Urological Association
Atlanta, Georgia / May 19-24, 2012
Atlanta - Overactive bladder (OAB) has a negative impact on health-related quality of life for millions of people. The OAB symptom complex includes urgency, frequency and nocturia, and a third of patients with OAB are incontinent. It may arise from detrusor overactivity or other types of urethrovesical dysfunction. Historically, treatment for OAB has relied primarily on anticholinergic agents, but they often cause uncomfortable side effects, while a substantial number of patients have inadequate symptom control. Recently, therapy for OAB with a neuromuscular agent has produced favourable results in small and single-centre trials. Here at the AUA, a randomized phase III clinical trial of this agent has substantiated previous studies by demonstrating significant improvement in all outcome parameters.
Chief Medical Editor: Dr. Léna Coïc, Montréal, Quebec
Anticholinergic agents have become the mainstay treatment for overactive bladder (OAB). However, many patients are inadequately controlled or cannot tolerate these agents.
Favourable results from smaller clinical trials in OAB with onabotulinumtoxinA have emerged. According to Dr. Victor Nitti, New York University, New York, this agent was shown to block acetylcholine release, leading to inhibition of efferent pathways of detrusor activity. It might also block afferent neurotransmitters and sensory pathways associated with OAB symptoms. To that end, investigators at 72 sites conducted a 24-week, randomized, placebo-controlled phase III study to evaluate its safety and efficacy in 557 patients with OAB inadequately controlled by anticholinergic therapy.
Investigators enrolled only patients with OAB associated with incontinence for a minimum of 6 consecutive months. Patients reported at least 3 urinary urgency episodes daily for 3 days, as documented in a patient diary. Patients reported ≥8 micturitions daily, a postvoid residual (PVR) ≤100 mL and inadequate symptom control or intolerable side effects with anticholinergics. Patients were randomized to intradetrusor injection of onabotulinumtoxinA or placebo injection. Patients received 20 injections of 0.5 mL/site. Retreatment with 100 U was permitted after 12 weeks.
The primary end points were number of urinary incontinence episodes and the proportion of patients who met criteria for positive treatment response by the Treatment Benefit Scale. Secondary end points included number of micturition episodes, number of urgency episodes, voided volume/micturition, Incontinence Quality of Life (I-QOL) score, and scores on role and social limitations domains of the King’s Health Questionnaire.
Major adverse events included urinary retention and urinary tract infection (UTI). Urinary retention was defined as initiation of clean intermittent catheterization in response to PVR ≥200 mL and <350 mL associated with symptoms or PVR ≥350 mL, regardless of symptoms or their absence. UTI was defined as a positive urine culture and bacteriuria count >105 CFU/mL and leukocyturia >5/hpf.
The duration of OAB averaged almost 7 years. Treatment history included a duration of 2.5 years with 2 to 3 different anticholinergics. The patients averaged >5 incontinence episodes daily, 12 micturition episodes daily, >8 urgency episodes daily and >2 nocturia episodes nightly. “This was a group of patients who had very severe OAB syndrome symptomatology,” Dr. Nitti told delegates.
Patients randomized to onabotulinumtoxinA had a mean reduction in daily incontinence episodes of 2.65 vs. 0.85 in the placebo group (P<0.001). The difference in treatment effect emerged within 2 weeks with a mean decrease of 2.85 episodes vs. 1.09 in the placebo group (P<0.001). The improvement in continence increased to an average reduction of 3.05 incontinence episodes compared with baseline at week 6 compared with 1.07 in the placebo group (P<0.001). “The difference in effect on incontinence emerged very early, and the difference in favour of the onabotulinumtoxinA group remained significant at every time point throughout the first 12 weeks,” noted Dr. Nitti.
More than half of the active treatment group had at least a 50% reduction in daily incontinence episodes vs. 28.9% of the placebo group (P<0.001) and 23% reported complete resolution of incontinence at 12 weeks vs. 6.5%, respectively (P<0.001). PVR declined by 32.6 mL with onabotulinumtoxinA and 2.5 mL with placebo (P<0.001).
The percentage reduction for each of the OAB-related outcomes showed significantly greater improvement from baseline in the actively-treated arm (Table 1). “Each of these differences represented statistically and clinically significant improvement in favour of treatment with onabotulinumtoxinA,” reported Dr. Nitti. “The difference ranged between three- and fourfold for each of the outcomes.” Secondary end points also demonstrated consistent and statistically significant improvement at week 12.
“A minimally important difference on the I-QOL is an improvement of ≥10 points, and the minimally important difference on the King’s Health Questionnaire is ≥5 points,” stated Dr. Nitti. “Patients treated with onabotulinumtoxinA had improvement that far exceeded the minimally important difference, whereas patients in the placebo group did not meet the criteria for minimal improvement.”
Table 1.
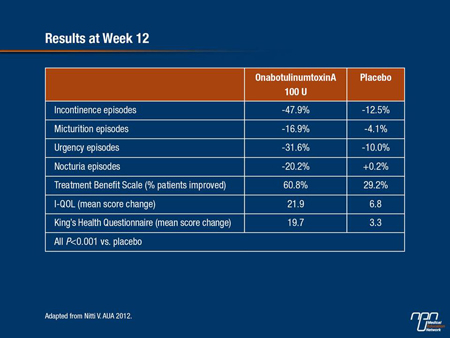
For each of the HR-QOL outcomes, significant differences in favour of active treatment emerged within the first 2 weeks and remained significantly different throughout the 12-week follow-up for primary and secondary end points. Each of the domains on the I-QOL demonstrated significant improvement in favour of active treatment (P<0.001 for all comparisons). The 6 domains of the Kings, Health Questionnaire improved more with onabotulinumtoxinA than with placebo (P<0.001 for all comparisons).
The most commonly reported adverse events during the first 12 weeks with onabotulinumtoxinA were UTI (15.5%), dysuria (12.2%), bacteriuria (5.0%) and urinary retention (5.4%). Hematuria occurred more often in the placebo arm (5.5% vs. 2.5%). Additionally, the rate of initiation of clean intermittent catheterization ranged from 4.7% at week 2 to 2.9% at week 12 in the onabotulinumtoxinA group; 6.1% of patients required clean intermittent catheterization at some point. No patient in the placebo arm required clean intermittent catheterization.
Overall discontinuation rates were 11.1% in the onabotulinumtoxinA arm and 12.3% in the placebo arm. Discontinuation because of adverse events was observed in 1.8% and 1.4%, respectively.
“Treatment with 100 U of onabotulinumtoxinA in OAB patients with urinary incontinence who were inadequately managed by anticholinergics results in significant and clinically relevant reductions in all OAB symptoms,” Dr. Nitti concluded. Patients assessed treatment as significantly improving their condition and QOL. Treatment had an acceptable safety profile with primarily localized adverse events.
Emerging Options
Results presented at the AUA meeting of the newer antimuscarinic fesoterodine showed significant improvement in OAB symptoms in patients 65 and older with urge incontinence. The placebo-controlled trial consisted of 552 patients with a mean age of 75 of which 50.4% were 75 or older. Patient score was ≥3 on the Vulnerable Elders Survey. Trial design included a flexible dosing regimen that permitted titration up or down in response to symptom relief and adverse effects (Abstract 1352).
Compared with baseline, after 12 weeks, treatment resulted in an average decrease of 2.84 incontinence episodes daily vs. 2.20 in the placebo group (P=0.0018). Mean daily micturition episodes decreased by 2.34 with fesoterodine and 1.50 with placebo (P=0.0003).
Several other treatments for OAB were reviewed by Dr. Masayuki Takeda, University of Yamanashi, Japan, who presented a review of highlights in urodynamics and female urology.
Presentations included a study of a transdermal gel formulation (applied once daily for 12 weeks) of the anticholinergic oxybutynin; 542 patients with OAB participated. Findings indicated significantly improved weekly incontinence episodes, daily frequency and voided volume with the gel formulation compared with placebo (Abstract 522).
Beta-3 adrenoreceptor agonists such as solabegron and mirabegron represent a new mechanistic approach to OAB treatment. In a phase II placebo-controlled trial involving 258 women with OAB, solabegron proved safe and reduced the number of incontinence episodes (Abstract 520). In a phase II study involving 200 men with lower urinary tract symptoms (LUTS) and bladder outlet obstruction, mirabegron showed no difference with placebo for any of the outcomes assessed (Abstract 1869). Conversely, long-term treatment with the neurostimulatory device demonstrated treatment success in 71% of 217 patients with OAB and LUTS refractory to initial treatment (Abstract 1956). After 47 months of follow-up, patients with idiopathic retention benefited more than did those with OAB (P=0.02).
Summary
Non-invasive treatment with antimuscarinics is a mainstay of therapy. Emerging oral beta-3 agents appear promising and may become an important addition in the management of OAB. For those for whom antimuscarinics are ineffective or who cannot tolerate them, onabotulinumtoxinA has significantly improved symptoms of severe, treatment-refractory OAB compared with placebo at 12 weeks. The treatment was well tolerated with similar discontinuation rates between treatment arms. A more invasive neurostimulatory device has a high success rate in patients refractory to initial treatment.