Reports
From the National Advisory Committee on Immunization
This report is based on medical evidence presented at sanctioned medical congress, from peer reviewed literature or opinion provided by a qualified healthcare practitioner. The consumption of the information contained within this report is intended for qualified Canadian healthcare practitioners only.
MEDI-NEWS - Update on Quadrivalent Conjugate Meningococcal Vaccines
March 2013
Ottawa - An update from the National Advisory Committee on Immunization (NACI) on quadrivalent conjugate meningococcal vaccines now includes recommendations for the use of the most recent of these vaccines, MenACWY-CRM197 and provides a template upon which health care professionals may base decisions regarding vaccination against invasive meningococcal disease (IMD) for specific age groups. According to this NACI update, 2 quadrivalent conjugate meningococcal vaccines, MenACWY-CRM197 and MenACWY-D are indicated in individuals between 2 and 55 years of age and both may be considered in individuals 56 years of age and older. Adolescents should now routinely receive a conjugate meningococcal vaccine around the age of 12 and either quadrivalent vaccine can be used. High-risk individuals should receive 2 doses of either vaccine, given 8 weeks apart. With certain exceptions, both quadrivalent meningococcal vaccines are equally recommended for those who require revaccination, but direct comparisons between the 2 indicate that MenACWY-CRM197 seems to evoke more robust immunological responses with higher rates of persistence. It is hypothesized that the higher rates of persistence of the immune response for MenACWY-CRM197 are related to the higher geometric mean titres that were achieved one month post-vaccination. In Canada, most IMD is caused by serogroups A, B, C, W-135 and Y, with serogroup B being the most common cause of IMD. A candidate vaccine against serogroup B IMD is also now showing considerable promise across all age groups.
Chief Medical Editor: Dr. Léna Coïc, Montréal, Quebec
Since 2001, the National Advisory Committee on Immunization (NACI) has recommended the use of the meningococcal C conjugate vaccine for infants under the age of 1 year, children between 1 and 4 years of age, adolescents and young adults. It may also be considered for children ≥5 years of age who have not yet reached adolescence.
Quadrivalent Conjugate Meningococcal Vaccines
The 2 available quadrivalent conjugate meningococcal vaccines MenACWY-CRM197 (Menveo) and MenACWY-D (Menactra) each contain serogroups A, C, W-135 and Y, but each serogroup in Menveo is individually conjugated to CRM197, a variant protein of diphtheria toxin while Menactra is conjugated to diphtheria toxoid. One dose of either vaccine is authorized for patients between 2 and 55 years of age and both may also be considered for patients 56 years of age or older.
In their latest update, NACI now recommends that adolescents around 12 years of age receive a conjugate meningococcal vaccine and either quadrivalent vaccine can be used. They also recommend that high-risk individuals 2 years of age and older receive 2 doses of either quadrivalent vaccine at least 8 weeks apart.
Importantly, Menveo is the recommended vaccine for high-risk children under two years of age. NACI defines high-risk as patients with underlying medical conditions including anatomic or functional asplenia (including sickle cell disease), those with complement, properdin, factor D or primary antibody deficiencies, and patients with acquired complement deficiency receiving eculizumab.
Research, industrial and clinical laboratory personnel who are routinely exposed to N. meningitidis should also receive a quadrivalent vaccine as well as travelers when it is recommended or required. For any individual at ongoing or recurring risk of exposure to meningococcal disease due to underlying medical conditions or to exposure, NACI recommends revaccination with either vaccine, regardless of which was used initially.
Based on expert opinion, NACI recommends that these individuals also receive a booster dose 3 to 5 years after the last dose if they were vaccinated at 6 years of age or under; this should be followed by a booster dose every 5 years. For those who were vaccinated at 7 years of age or older, NACI recommends a booster dose 5 years after the last dose, followed by a booster every 5 years.
NACI similarly recommends either quadrivalent vaccine be given to close contacts of patients with IMD caused by serogroups A, C, W-135 or Y, as well as for the control of outbreaks caused by the same serogroups. Either vaccine may be used in children 2 years of age and older. However, for close contacts of patients with IMD who are under the age of 2, NACI recommends only Menveo be used. Protection from vaccination post-exposure to IMD should last at least one year as this is the period where household contacts remain at increased risk.
Noteworthy as well, Menveo has been co-administered with many other routine pediatric vaccines and no immunological interference has been observed.
For Prevention and Protection
As NACI suggests, health care providers need to emphasize that although rare, IMD still carries a mortality rate of about 10% and 10 to 20% of survivors have long-term sequelae including hearing loss, neurologic disabilities, and digit or limb amputations. Both quadrivalent vaccines are equally recommended for the prevention of IMD caused by 4 out of the main 5 serogroups. In every comparison assessing serogroups C, Y and W-135, and in most comparisons assessing serogroup A, Menveo was found to be non-inferior and in several instances to have a statistically superior immune response to Menactra. It is hypothesized that the higher rates of persistence of the immune response for Menveo are related to the higher geometric mean titres (GMTs) that were achieved one month post-vaccination.
Adolescents
A phase III study in 2180 adolescents between 11 and 18 years of age by Jackson et al. (Clin Infect Dis 2009;49(1):
e1-e10) found that GMTs one month following vaccination were superior with Menveo than with Menactra for all serogroups except serogroup C, where Menveo was non-inferior to Menactra (Figure 1).
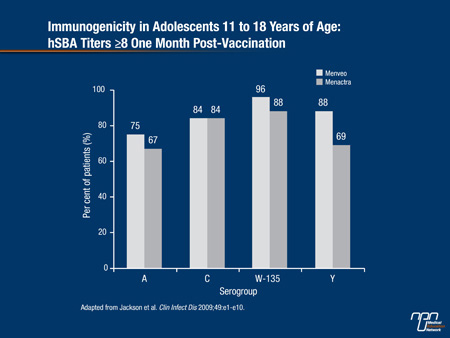
The greatest difference in the percentage of adolescents achieving an hSBA titre of ≥8 was found for serogroup Y: 88% for Menveo vs. 69% for Menactra.
At a median of 22 months post-vaccination, Gill et al. (Human Vaccines 2010;6(11):1-7) found that GMTs in a subset of adolescents enrolled in the Jackson et al. study were significantly higher for Menveo recipients for serogroups A, W-135 and Y and non-significantly higher for serogroup C. For the Menveo group, the percentage of patients who achieved hSBA titres of ≥8 at a median of 22 months post-vaccination were 36% against serogroup A, 62% against serogroup C, 84% against W-135 and 67% against serogroup Y.
2 to 10 Years of Age
A study by Halperin et al. compared Menveo to Menactra in 2907 children stratified by age: 2 to 5-year olds and 6 to 10 year-olds (Vaccine 2010;28(50):7865-72). Some of the 2-5 year-olds received 2 doses of the MenACWY-CRM197 vaccine given 60 days apart. At 1 month post-vaccination, hSBA titres for both age groups showed that the percentage of children who received Menveo and who achieved a titre of ≥8 was lower for serogroup A, non-inferior for serogroup C and significantly superior for serogroups W-135 and Y compared to children who received Menactra.
The percentage of children in both age groups achieving protective antibody titres one month after vaccination was lower for serogroup A and higher for all other serogroups for Menveo compared to Menactra as well.
Comparison of GMTs for both age groups combined also demonstrated Menveo was non-inferior to Menactra for serogroup A and significantly superior for serogroups C, W-135 and Y. The 2-5 year olds who received 2 doses of Menveo had much higher GMTs for all serogroups and a higher percentage achieved a titre of ≥8 for all serogroups.
When both age groups were combined, Menveo was non-inferior to Menactra for all 4 serogroups and significantly superior for groups C, W-135 and Y. The safety profile of both vaccines was similar across both age groups, suggesting that both vaccines are immunogenic and well tolerated in children between 2 and 10 years of age.
Summary
Canada currently has 2 quadrivalent conjugate meningococcal vaccines that are recommended for the prevention of IMD caused by 4 out of the 5 most pathogenic N. meningitidis serogroups in individuals between 2 and 55 years of age. The quadrivalent conjugate vaccines may be additionally considered in individuals 56 years of age and older. A new vaccine is on the horizon that will help prevent serogroup B disease, and when available these vaccines should help to significantly reduce IMD in all vulnerable age groups, most notably in infants, toddlers and adolescents.
NACI guidelines update at: http://www.phac-aspc.gc.ca/publicat/ccdr-rmtc/13vol39/acs-dcc-1/assets/pdf/acs-dcc-4-eng.pdf