Reports
IMPROVE-IT: Reaffirmation of Lipid Hypothesis By Non-Statin Ezetimibe in High-Risk Population
This report is based on medical evidence presented at sanctioned medical congress, from peer reviewed literature or opinion provided by a qualified healthcare practitioner. The consumption of the information contained within this report is intended for qualified Canadian healthcare practitioners only.
PHYSICIAN PERSPECTIVE - Viewpoint based on presentations from the American Heart Association (AHA) 2014 Scientific Sessions
Chicago, Illinois / November 15-19, 2014
Guest Editor:
Dr. Paul W. Armstrong, MD, FCAHS, FRSC
Distinguished University Professor
Mazankowski Alberta Heart Institute
University of Alberta
Edmonton, Alberta
Dr. Armstrong served on the Steering Committee of IMPROVE-IT and was one of three national IMPROVE-IT lead investigators for Canada
Introduction
In the recently completed IMPROVE-IT trial, adding ezetimibe to simvastatin in a high-risk population with a recent prior acute coronary event significantly reduced the risk of cardiovascular (CV) events relative to simvastatin alone. It is the first trial in the management of dyslipidemia to show additional CV risk reduction from adding a non-statin to a statin. The improvement in outcome was achieved even though those on simvastatin were at the currently recommended treatment goal. These data reinforce the proportional relationship between reductions in LDL-C and protection from CV events in high-risk patients, which is the basic premise of the lipid hypothesis. In addition, they demonstrate that even greater CV risk reductions can be achieved by a further lowering of LDL-C below the currently recommended goal. The results are relevant to a better understanding of the relationship between LDL-C and CV risk and to clinical practice.
The data generated by IMPROVE-IT (IMProved Reduction of Outcomes: Vytorin Efficacy International Trial) supply an evidence basis for adding ezetimibe to a statin to reduce CV risk. Current guidelines, including those from the Canadian Cardiovascular Society (CCS) (Anderson TJ et al.Can J Cardiol 2013;29:151-67), state that no therapy added to statins has been associated with a significant reduction in CV events. This statement now deserves to be revisited given that IMPROVE-IT provides the evidence that LDL-C lowering with ezetimibe in a high-risk population produces additional risk reduction commensurate with the reduction in LDL-C. In an extended follow-up, IMPROVE-IT also demonstrated that ezetimibe is both safe and well tolerated.
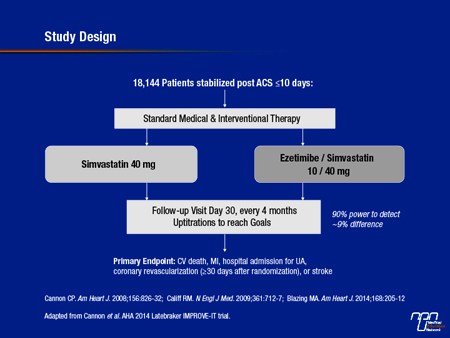
In the double-blind IMPROVE-IT trial, 18,144 patients were enrolled within 10 days of hospitalization for an acute coronary syndrome (ACS) (see Figure 1). They were randomized at 1158 participating centres in 39 countries, including Canada, to receive 40 mg simvastatin or the same dose of simvastatin plus 10 mg of ezetimibe. Uptitration of the statin was permitted. The goal was to achieve LDL-C of <1.8 mmol/L. The primary endpoint was relative reduction in CV death, myocardial infarction (MI), stroke, or hospital admission for unstable angina or a coronary revascularization (ordered at least 30 days after enrolment).
Lower LDL-C = Lower CV Risk
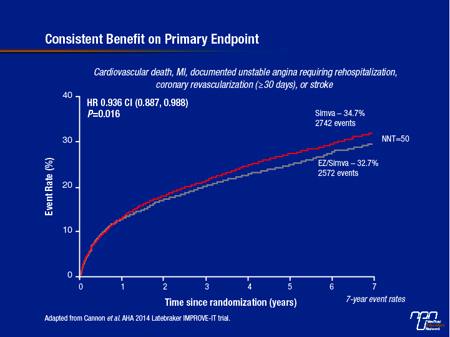
On the intention-to-treat (ITT) analysis, the hazard ratio (HR) for the primary endpoint favouring the combination of ezetimibe and simvastatin (EZ/S) was 0.936 (95% CI, 0.887–0.988; P=0.016) after a median follow-up of approximately 6 years (see Figure 2). Similar relative and statistically significant protection was observed for all three of the prespecified secondary endpoints, which evaluated various combinations of events included in the primary endpoint. These ranged from about a 5% risk reduction for all CV deaths, MI, unstable angina, all revascularizations, and stroke to nearly 9% for CV death, MI, and urgent coronary revascularization. For the combined endpoint of CV death, MI, or non-fatal stroke endpoint, the event rate was 20.4% on EZ/S versus 22.2% on simvastatin alone for a 10% risk reduction (HR 0.90; 95% CI: 0.84-0.97; P=0.003) and a number needed-to-treat of 56.
Figure 1
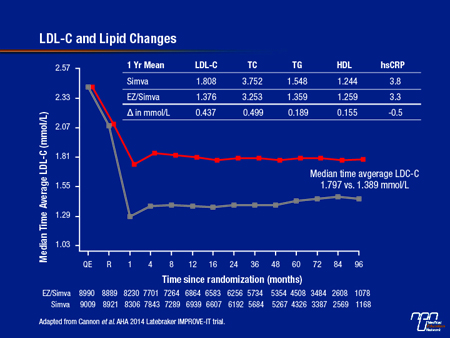
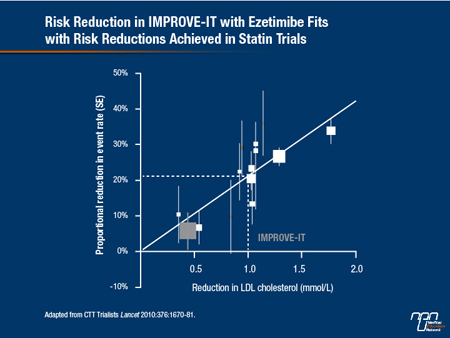
Although these risk reductions appear modest, their clinical significance is meaningful when viewed in the context of the trial design, which achieved rigorous control of hyperlipidemia in patients receiving simvastatin alone. As a result of the uptitration scheme, the median LDL-C in the simvastatin arm at the end of both 1 year and 5 years of follow-up was approximately 1.8 mmol/L. In patients randomized to EZ/S, the median LDL-C was further reduced to 1,389 mmol/L (see Figure 3). This relative 20% additional reduction in LDL-C produced an incremental CV event risk reduction consistent with that predicted by the Cholesterol Treatment Trialists (CTT) Collaboration from statin trials (Lancet 2010;376:1670-81) (see Figure 4). The validation of the proportional risk reduction with a non-statin strengthens the rationale for the use of this therapy in those who do not reach goals on a statin alone. It also underscores the need for long-term follow-up in such studies. Hence, it took time for the benefits to accrue with the impact on event reduction first evident after 1 year and increasing over time thereafter.
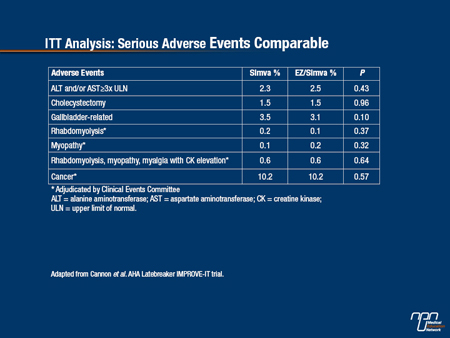
IMPROVE-IT also provided a large body of data with which to validate the safety of ezetimibe (see Table 1). In data drawn from both the ITT analysis, which included more than 80,000 patient-years of follow-up for the primary endpoint, and the pre-specified exploratory on-treatment (OT) analyses, which included more than 60,000 patient-years of follow-up, no adverse safety signals were observed, including areas of particular interest, such as gallbladder-related events, myopathies, liver function, or cancer. These data indicate that the additional CV risk reductions produced with the addition of ezetimibe to a statin are achieved without a safety hazard.
Table 1.
On-Treatment Analysis: Larger Effect
The pre-specified OT analysis addressed the potential for off-treatment events to dilute the power of IMPROVE-IT to accurately capture the efficacy and safety of EZ/S relative to simvastatin. In this analysis, data were censored a minimum of 30 days after the last dose or at the last complete endpoint ascertainment of clinical events. Of the 17,706 patients who received at least one dose of therapy after randomization, 10,573 were followed for a mean of 4.4 years on their assignment therapy and 7,133 patients were followed off drug according to their original arm assignments.
In the OT analysis, LDL-C reductions and CV risk reductions in both study arms were slightly greater than that observed in the ITT analysis. On EZ/S, the relative reduction in LDL-C at 1 year climbed to 0.44 mmol/L, bringing the median LDL-C in the EZ/S arm to <1.3 mmol/L. Consistent with the expected correlation between LDL-C lowering and event reduction, the slightly greater control of lipids was reflected in slightly greater reduction in the primary endpoint (HR 0.924; 95% CI: 0.863-0.983; P=0.012) and pre-specified secondary endpoints for EZ/S versus simvastatin alone.
Ezetimibe reduces LDL-C by reducing cholesterol absorption from the gastrointestinal (GI) tract through inhibition of the Niemann-Pick C1-like 1 (NPC1L1) protein, which is a mediator of this uptake. The LDL-C reductions achieved with ezetimibe in the ITT and OT IMPROVE-IT analyses are consistent with a large number of previous studies conducted with this therapy. Although ezetimibe has long been approved as an adjunctive therapy or substitute for statins in patients who have not reached LDL-C treatment goals, the IMPROVE-IT trial is the first to confirm that this produces the expected additional risk reduction.
Figure 2
Lipid Hypothesis Reinforced
The data also reinforce the lipid hypothesis, which preceded the introduction of statins. The evidence that LDL-C is a treatable risk factor dates back to 1984 when the Lipid Research Clinics Coronary Primary Prevention trial (LRC-CPPT) attributed a CV risk reduction to a reduction in LDL-C with the bile acid sequestrant cholestyramine (LRC-CPPT Investigators. JAMA 1984;251:351-64). Like ezetimibe, cholestyramine, which is less well tolerated, also produces about a 20% reduction in LDL-C. The modern era of lipid lowering guidelines was initiated in the 1990s with the introduction of statins. In a series of secondary and primary prevention trials, additional reductions in LDL-C were associated with incremental reductions in risk. Current treatment goals are based on those statin trials.
Figure 3.
Until IMPROVE-IT, it had been hypothesized — but not proven — that lipid lowering with therapies other than statins would provide similar reductions in CV risk. Over the 9 years since planning for the IMPROVE-IT trial began, the demand for proof has only intensified. One issue arises from the pleiotropic actions of statins, such as their anti-inflammatory effects that are not shared by other agents that reduce LDL-C. Concern regarding assumptions about cholesterol biology was also raised by the series of negative trials with agents effective at raising HDL-C. In addition, two prior ezetimibe trials failed to meet their primary endpoint. In the ENHANCE trial, the reduction in carotid intima media thickness (a surrogate endpoint) on ezetimibe relative to control in patients with familial hypercholesterolemia fell short of statistical significance (Kastelein et al. N Engl J Med 2008;358:1431-43). In SEAS, the combination of ezetimibe and simvastatin did not reduce ischemic events in patients with aortic stenosis (Rossebo et al. N Engl J Med 2008;359:1343-56). Although neither result precluded the ability of ezetimibe to achieve a CV risk reduction in high-risk patients though lipid lowering, the IMPROVE-IT data confirm this effect and provide reassurance of the validity of the underlying study hypothesis.
Even Lower LDL-C Is Even Better
Of the data generated by IMPROVE-IT, the evidence that additional LDL-C lowering beyond that currently recommended in the current CCS guidelines may be the most important. These data demonstrate that additional protection from CV events can be safely achieved with additional reductions in LDL-C at least to the mean of 1.4 mmol/L achieved in the EZ/S group. The finding is consistent with post-hoc analyses from several major statin trials, such as TNT, PROVE-IT, and JUPITER. Post-hoc analyses of patients who achieved very low LDL-C levels in these studies have yet to identify a level of LDL-C below which there is no further CV risk reductions or a level at which safety issues have been identified.
IMPROVE-IT does not establish the optimal target of LDL-C for high-risk patients. Benefit may still accrue from even lower levels than those achieved on average in the EZ/S arm of the trial. However, the additional risk reduction in the absence of an increased risk of adverse events from the greater LDL-C lowering in the experimental arm does establish a new goal. IMPROVE-IT both reaffirms and extends the lipid hypothesis by demonstrating added benefit from LDL-C reductions below current goals. Specifically, IMPROVE-IT demonstrates that ezetimibe added to a statin provides an incremental reduction in CV risk proportional to its ability to lower LDL-C.
Figure 4.
Conclusion
IMPROVE-IT represents a milestone in the on-going effort to reduce the risk of CV events. By proving that additional LDL-C reductions with ezetimibe over a statin alone provides additional CV risk reductions, it expands the evidence base for a treatment option. As the first trial to demonstrate this effect with a non-statin, IMPROVE-IT also substantially reinforces the lipid hypothesis. Data from this trial redefine optimal LDL-C control in very high-risk populations and encourage further study to evaluate the potential of still lower LDL-C levels to confer even greater CV risk protection. IMPROVE-IT establishes the next step, but likely not the last advance, in the on-going effort to achieve optimal CV risk reductions through lipid lowering.