Reports
Invasive Meningococcal Disease in Infants: Immunogenicity and Safety of a Novel Multi-Component Serogroup B Vaccine
This report is based on medical evidence presented at sanctioned medical congress, from peer reviewed literature or opinion provided by a qualified healthcare practitioner. The consumption of the information contained within this report is intended for qualified Canadian healthcare practitioners only.
MEDI-NEWS - Based on Lancet 2013;381(9869):825-35.
Serogroup B is now the dominant cause of invasive meningococcal disease (IMD) in Canada where it disproportionally affects infants. This underscores the importance of having an effective vaccine for this vulnerable age group. A multi-component vaccine against serogroup B has now been evaluated in a large cohort of infants. Results showed that the multi-component vaccine was immunogenic in infants from 2 months of age onwards and that it did not significantly interfere with the immunogenicity of other routine infant vaccines when given concomitantly. Responses to the vaccine were also robust both to the primary series as well as a booster dose given at 12 months. Rates of medical attention for fever that predictably occur 6 hours post-vaccination were low. The study demonstrates that the novel multi-component vaccine is immunogenic in a population at highest risk of serogroup B IMD.
Chief Medical Editor: Dr. Léna Coïc, Montréal, Quebec
Out of the 5 main bacterial serogroups that cause invasive meningococcal disease (IMD), serogroup B disproportionately affects young infants and is now the most common cause of infant bacterial meningitis and septicemia in Europe and Canada. According to the National Advisory Committee on Immunization (NACI), in 2007, over 80% of IMD in infants under the age of 1 year was caused by serogroup B (Update on the use of Quadrivalent Conjugate Meningococcal Vaccines. Canada Communicable Disease Report, January 2013; Vol. 39, ACS-1). Serogroup B causes the highest incidence of IMD in Canada and children <1 year of age suffer the greatest disease burden. (Bettinger et al. PIDJ. 2013;32(1): e20-e25). Available vaccines are not able to provide protection against serogroup B; as such, the burden of illness caused by this serogroup remains an unmet medical need.
The traditional polysaccharide conjugate-approach used to develop effective vaccines against serogroups A, C, Y and W-135 could not be used for the development of a vaccine for serogroup B because the capsule that characterizes this particular serogroup is immunologically similar to neural-cell adhesion molecules and is thus poorly immunogenic.
Using a technique called “reverse vaccinology”, scientists eventually identified 3 primary recombinant antigens deemed appropriate for a serogroup B vaccine: factor-H-binding protein (fHbp), Neisserial adhesin A (NadA) and Neisserial-heparin-binding antigens (NHBA). Early studies showed that these antigens, combined with an outer membrane vesicle of a strain of serogroup B that had caused an outbreak in New Zealand, were bactericidal in vitro. This multicomponent meningoccal B vaccine (4CMenB) represents the first ever MenB vaccine designed to cover different strains of menB around the world.
The clinical development program supporting the safety, tolerability and immunogenicity of 4CMenB has spanned all ages involving over 7800 subjects, 5850 infants and toddlers and 1712 adolescents and adults across 13 different clinical studies. In 1 pivotal phase III study, the 4CMenB vaccine was administered to infants at 2, 4 and 6 months of age with a booster dose given at 12 months of age. It was also given with and without routine infant vaccines to ensure these remained immunogenic when given concomitantly. A total of 3630 infants were enrolled in the study—2627 were randomized to an open-label immunogenicity substudy and 1003 were randomized to an observer-blinded safety substudy.
Of those randomized to the immunogenicity study, 1968 were allocated to routine infant vaccines plus the 4CMenB vaccine while 659 were allocated to routine infant vaccines alone. In the safety substudy, 533 infants were allocated to routine infant vaccines plus the 4CMenB vaccine while 490 infants received routine infant vaccines plus a meningococcal C vaccine.
Parents whose infants completed the primary series in the immunogenicity or the safety substudy were invited to enroll their child in the booster phase of the study at 12 months of age. Children received either the 4CMenB alone as a booster at 12 months (n=789) or together with the measles/mumps/rubella/varicella (MMRV) vaccine (n=766).
Immunogenicity was assessed by serum bactericidal assay with human complement (hSBA) against serogroup B test strains, where an hSBA titre ≥5 correlated with protection.
Immunogenicity and Safety
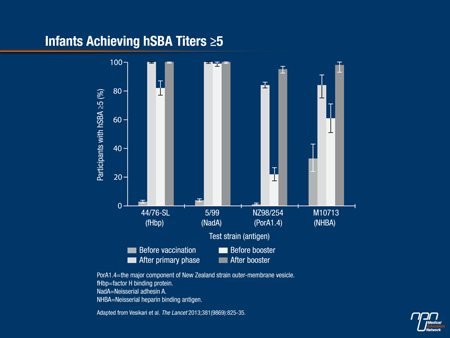
As reported by Vesikari et al. (The Lancet 2013;381(9869):825-35), 100% of infants achieved an hSBA titre of ≥5 against fHbp, NadA, NHBA indicator strains one month after receiving all 3 doses of the vaccine, while 84% achieved the same hSBA titre against the New Zealand outer-membrane vesicle indicator strain (Figure 1). A subset of 100 infants was tested post-hoc for responses to the NHBA strain and 84% obtained an hSBA titre of ≥5. As the authors pointed out, antibody responses to the different strains waned by the age of 12 months.
Following a booster dose either given alone or concomitantly with the MMRV vaccine, a booster response was observed against all test strains, resulting in hSBA titres of ≥5 against all 4 vaccine antigens in 95% to 100% of recipients. With the exception of infant response to poliovirus type 2, immunological responses to all constituent antigens reached an acceptable level when routine vaccines were given concomitantly with the 4CMenB vaccine.
Figure 1.
Pooled safety data for all doses combined indicated that injection site reactions peaked on day 1 but dropped steeply on day 2 and were similar whether infants received the 4CMenB vaccine alone or with routine infant vaccinations. Following 4CMenB booster doses, the occurrence of injection site reactions was much lower. The most notable systemic reaction in both infants and children at 12 months was fever, typically peaking 6 hours after vaccination whether given alone or concomitantly with routine infant vaccines.
The need for medical attention to treat fever was low (2 to 3% of doses) and for medical procedures was even lower at 1% when the 4CMenB vaccine was given in combination with routine infant vaccines vs. 1.1% when routine vaccines were given alone and 1% when routine vaccines were given together with the meningococcal C vaccine. Following the booster dose, fever rates did not differ among infants given the 4CMenB vaccine alone or with the MMRV vaccine.
Future Considerations
In an accompanying editorial, Dr. Matthew Snape and Dr. Andrew Pollard, Oxford Vaccine Group, University of Oxford and the NIHR Oxford Biomedical Research Centre, UK, respectively, asked whether or not the 4CMenB vaccine might be the “beginning of the end” for IMD caused by serogroup B Neisseria meningitidis. The relative rarity of IMD caused by serogroup B means that vaccine approval will have to be made on the basis of bactericidal antibody levels against strains contained in the vaccine rather than direct evidence it prevents serogroup B disease.
Conversely, physicians can be confident of the vaccine’s partial effectiveness, as the editorialists pointed out, since 1 of the 4 components contained in the vaccine is identical to the New Zealand MenB vaccine that successfully controlled a clonal outbreak in New Zealand.
The same B strain has been circulating in Europe over the past 2 decades as well. The proportion of serogroup B strains susceptible to the 4CMenB vaccine remains to be elucidated. As assessed by Donnelly et al. in 2011 during the 11th European Meningococcal Disease Society in Slovenia, immune responses raised by the vaccine suggest that the 4CMenB vaccine will protect against 77.5% of IMD caused by serogroup B N. meningitidis across 5 European countries. According to the authors, if these estimates are correct, control of meningococcal disease could be imminent.
For those working on the development of a vaccine against serogroup B disease over the past 40 years, the size of the step forward with the imminent licensure of the 4CMenB vaccine is obvious, as the authors suggested.
In an interview, Dr. Ronald Gold, Professor of Pediatrics (retired), University of Toronto, Ontario, commented, “It’s a very promising vaccine. It produces what we consider to be protective antibodies in all age groups starting at 2 months of age.”
As the Vesikari et al. study showed, the 4CMenB vaccine, as a separate injection, can be given without interfering with the immunogenicity of concomitant routine infant vaccines.
Concomitant administration with routine infant vaccines similarly does not enhance its reactogenicity profile or that of other vaccines and studies indicate there is no excess of physician visits for adverse events when 4CMenB is given with other vaccines.