Reports
Iron Chelation in Hematological Disorders: Emerging Data on Non-Transfusion-Dependent Thalassemia and Myelodysplastic Syndrome
This report is based on medical evidence presented at sanctioned medical congress, from peer reviewed literature or opinion provided by a qualified healthcare practitioner. The consumption of the information contained within this report is intended for qualified Canadian healthcare practitioners only.
MEDICAL FRONTIERS - 17th Congress of the European Hematology Association
Amsterdam, The Netherlands / June 14-17, 2012
Amsterdam - Iron overload is a complication found in a range of hematological disorders. While it is most strongly associated with transfusion dependence, it can also result from the disease process itself, notably in non-transfusion-dependent thalassemia. This patient group is now recognized as having comparable iron burden to those with transfusion-dependent thalassemia major. In patients with transfusion-dependent myelodysplastic syndrome (MDS), evidence for the benefits of chelation for survival continues to accumulate. Emerging data also suggest that chelation may potentially modify the course of MDS by increasing the likelihood of hematological response, although further studies will be needed to confirm this intriguing hypothesis.
Non-transfusion-dependent thalassemia (NTDT) is group of thalassemias where patients have a limited or no requirement for regular blood transfusions. Patients may require occasional transfusions in specific situations including growth failure, pregnancy or during infections. It has 3 predominant forms: hemoglobin H (HbH) disease, HbE/beta-thalassemia and beta-thalassemia intermedia. Despite advances in molecular and laboratory techniques, diagnosis is still primarily clinical, and the disease can be difficult to recognize. Mild disease can be asymptomatic for long periods and may not be diagnosed until adulthood. More severe disease manifests in childhood. Correct diagnosis is vital to prevent children being placed unnecessarily on lifelong transfusion therapy.
“The clinical characteristics of NTDT are driven by ineffective erythropoiesis, hemolysis and iron overload,” stated Dr. Bart Biemond, Academic Medical Center, Amsterdam, The Netherlands. Symptoms include anemia, extramedullary hematopoiesis, splenomegaly and manifestations of chronic hemolysis such as leg ulcers, jaundice, pulmonary hypertension and thromboembolic events. Iron overload results from ineffective hematopoiesis and increased gut iron absorption, and causes endocrinopathies and liver disease. In children, iron-related endocrine disruption and the effects of anemia can act together to lead to short stature and delayed pubertal development.
“The complications of NTDT increase with age,” noted Dr. Ali Taher, American University of Beirut Medical Center, Lebanon. Iron burden also increases over time and affects morbidity: high serum ferritin is a risk factor for thromboembolic events and pulmonary hypertension is correlated with liver iron concentration (LIC). Beta-thalassemia intermedia patients can develop severe iron overload in the liver if left untreated. “These patients can have similar LIC to transfused thalassemia major patients of the same age,” Dr. Taher told EHA delegates.
Blood transfusions are beneficial for many patients but add to iron burden. In young patients, they aim to suppress bone marrow activity, promoting growth and decreasing gut iron absorption. Blood transfusions are also thought to be protective against many of the later complications of NTDT.
Iron Chelation in NTDT
“Transfusion dependency varies widely from never required to almost full dependency,” noted Prof. Maria Domenica Cappellini, University of Milan, Italy. “NTDT patients need to be closely monitored for iron overload,” she emphasized. Monitoring needs to be more detailed than with thalassemia major because NTDT patients do not have a defined monthly iron intake.
There is no clear consensus on when to initiate iron chelation. Previous guidelines have suggested an LIC of ≥7 mg/g dw, but we now know that this is the threshold for complications to arise, Prof. Cappellini told delegates. The recommendation will therefore need to be revisited. A recent study published by Dr. Taher found that iron chelation is protective against pulmonary hypertension, hypogonadism, osteoporosis and cholelithiasis (Blood 2010;115(10):1886-92).
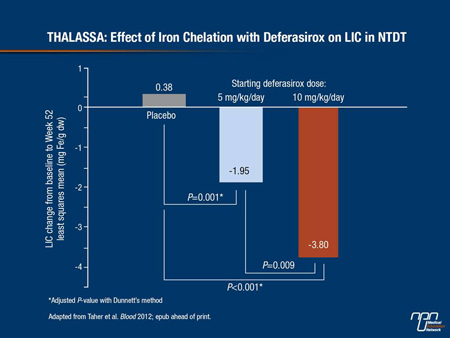
The efficacy and safety of chelation with deferasirox in NTDT was assessed in the THALASSA study (Taher et al. Blood 2012; epub ahead of print), which was reported in December 2011. THALASSA included 166 patients with NTDT aged 10 years and over, with LIC ≥5 mg Fe/g dw and serum ferritin >300 ng/mL. “We were struck by the very high LIC that these patients had at baseline,” remarked Prof. Cappellini. This was particularly surprising as the trial excluded patients with a regular transfusion requirement. Patients were randomized to starting doses of 5 or 10 mg/kg/day or matching placebo. A 10 mg/kg/day starting dose significantly reduced LIC from baseline by 3.8 mg/g dw, compared to an increase of 0.38 mg Fe/g dw in patients on placebo (P<0.001) (Figure 1). Adverse events were similar in the active treatment and placebo arms. The most common adverse events reported were rash and gastrointestinal upset, which was manageable.
Figure 1.
Managing NTDT: Treatment and Monitoring
How should NTDT patients be managed in clinical practice? The wide variations in severity and clinical presentation make clear treatment guidelines difficult, Prof. Cappellini confirmed. Treatment options include splenectomy, induction of fetal hemoglobin (HbF) using hydroxyurea, blood transfusions and iron chelation. Splenectomy is now less commonly performed due to the increased risk of complications in splenectomized patients. HbF induction is controversial: treatment has to be lifelong but may protect against complications.
One thing is clear: NTDT patients need close and regular follow-up. Prof. Antonis Kattamis, University of Athens, Greece, expanded on the theme of monitoring in NTDT. Monitoring has 3 components: monitoring anemia, monitoring for disease complications and monitoring iron overload. “There are several ways to assess total body iron and each has its advantages and disadvantages,” Prof. Kattamis explained. LIC by MRI is becoming standard in thalassemias. LIC can also be measured by biopsy or by a superconducting quantum interference device (SQUID). The degree of correlation between serum ferritin and LIC varies depending on the underlying disease, he noted. In beta-thalassemia intermedia, it underestimates LIC. However, a new analysis presented here at the EHA by Dr. Taher’s group showed that serum ferritin of >800 ng/mL is highly predictive of LIC ≥5 mg/g dw in NTDT and is therefore a useful monitoring tool.
NTDT patients should have serum ferritin measured every 6 to 12 months, Prof. Kattamis recommended. LIC should be measured at least once if there are signs of iron overload, and then every 1 to 2 years thereafter. Use of transferrin saturation, non-transferrin-bound iron (NTBI) and labile plasma iron (LPI) remain experimental.
Myelodysplastic Syndrome
MDS can occur at any age but predominantly affects patients aged 70 and over. Choice of therapy is driven by prognostic score, with patients generally classified as either lower risk (International Prognostic Scoring System low and intermediate-1 categories) or higher risk. The elderly nature of the population, and the correspondingly high levels of comorbidity, are one reason for the lack of randomized, controlled trials of iron chelation in MDS, noted Prof. Theo de Witte, Radboud University Medical Centre, Nijmegen, The Netherlands.
Despite use of disease-modifying therapies and agents to treat anemia as appropriate, around 85% of patients will eventually become transfusion-dependent and at risk of iron overload. Strategies to manage iron overload are particularly important in lower-risk MDS, where patients typically live with regular blood transfusions for a number of years.
Update from the EU MDS Registry
Much of our knowledge about the effects of iron toxicity on clinical outcomes in MDS comes from registry data. The EU MDS registry, set up by the European LeukemiaNet, is a valuable new source of prospective information on 1000 lower-risk patients from 14 European countries. By including only newly diagnosed patients, EU MDS avoids the risk of selection bias. While the focus is on epidemiology and disease management, the registry also collects outcomes data in relation to transfusion dependency, iron overload and iron chelation. Here at the EHA, Prof. de Witte presented recently published data from the first 18 months of follow-up.
Transfusion-dependent patients (29% of the sample) had a significantly higher mortality rate at 18 months (21% compared with 5% in the non-transfusion dependent group, P<0.0001). Transfusion-dependent patients with baseline serum ferritin ≥1000 µg/L had significantly higher mortality than those with serum ferritin <300 µg/L (HR 3.93 [1.98-7.78], P<0.0001). Although transfusion dependency is a marker of disease severity, it is also an independent prognostic factor for survival, and serum ferritin may also be an independent prognostic factor in transfusion-dependent patients, Prof. de Witte confirmed. So far, only 55 patients in the registry have received iron chelation.
Iron Chelation Effect on Survival
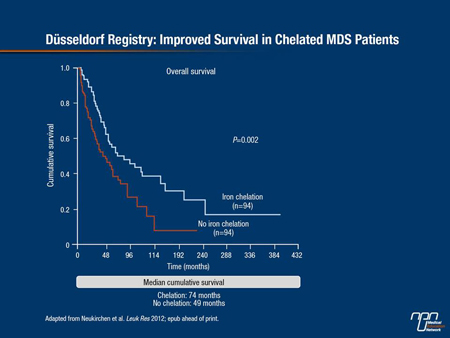
Numerous studies have shown that iron overload, as measured by high serum ferritin, is associated with reduced survival in MDS. Evidence is also mounting that iron chelation may prolong survival in transfusion-dependent MDS patients. At the EHA, Dr. Judith Neukirchen, Heinrich-Heine University, Düsseldorf, Germany, and colleagues presented new data on the impact of transfusion and chelation from the German Düsseldorf registry, the largest European MDS database. The retrospective analysis was carried out on 417 patients who had been assessed for serum ferritin. Transfusion-dependent patients (n=374) were considered to be chelated if they had received iron chelation for a total of at least 6 months: 85 patients met this criterion. Median survival was 67 months in the chelated group compared with 30 months in non-chelated transfusion-dependent patients (hazard ratios and P values not reported). In press from the Düsseldorf registry is a matched pair analysis on 94 chelated and 94 non-chelated patients. Again, chelated patients had longer survival (median cumulative survival 74 months vs. 49 months in matched non-chelated patients, P=0.002) (Figure 2).
Figure 2.
“There is much interest in whether iron chelation is just part of best supportive care or whether it is modifying the history of the disease in some way, but we need prospective data to answer this question,” stated Prof. Valeria Santini, University of Florence, Italy. This will be provided by TELESTO, a randomized, double-blind, placebo-controlled trial of iron chelation with deferasirox in 630 patients with lower-risk MDS. Patients are randomized 2:1 to active treatment or placebo, and follow-up will last for 5 years. Entry requirements include serum ferritin >1000 µg/L and a transfusion history of 15-75 units of packed red blood cells. The primary end point is event-free survival (death, and non-fatal cardiac and hepatic events).
In anticipation of TELESTO results, the question arose whether these patients should be offered chelation. Prof. Santini responded that in her practice, the decision is tailored to the patient’s comorbidities but in general, chelation is offered. Patients in the placebo arm of the study can be chelated if signs of iron toxicity arise, she emphasized.
Study Findings
“Part of the survival benefit from iron chelation may be attributable to improved bone marrow function,” according to Prof. Norbert Gattermann, Heinrich-Heine University. “There may be a vicious cycle in which iron overload negatively affects bone marrow function which in turn increases transfusion dependency and iron burden.”
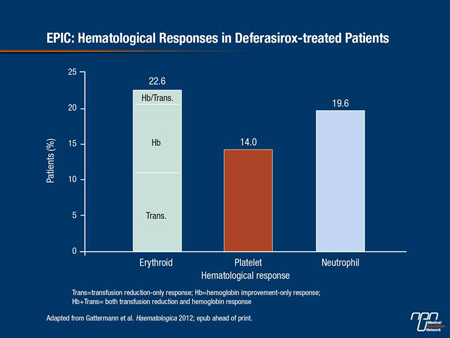
There is increasing evidence that iron chelation can improve hematological parameters in a subset of MDS patients, Prof. Gattermann continued. This mainly involves erythroid responses, although platelet and neutrophil responses have also been reported. The numerous case reports and small series in the literature are now supported by data from the EPIC and US03 trials. A post-hoc analysis of the EPIC study—the largest trial to monitor the efficacy of deferasirox in reducing iron burden in a range of hematological disorders—showed that about 20% of patients achieved an erythroid response (Figure 3). There was a trend towards greater reduction in serum ferritin in responders, but the difference did not reach statistical significance. The US03 trial has also recently reported encouraging hematopoietic responses with deferasirox: 15% of patients achieved erythroid and neutrophil responses and 22% achieved platelet response. Here at the EHA, Improta et al. reported that in a series of 55 iron-overloaded MDS patients treated with deferasirox, around a third had a reduced transfusion requirement after 2 years of treatment (abstract 0350).
Figure 3. EPIC: Hematological Responses in Deferasirox-treated Patients
Several potential mechanisms for this effect have been proposed, but Prof. Gattermann remarked that a reduction in iron-related oxidative stress was the most attractive hypothesis. Excess oxidative stress has been documented in MDS, he noted, but it is difficult to know what proportion of this is attributable to iron overload. However, data published by Ghoti et al. (Haematologica 2010;95(8):1433-4) showed reductions in a range of oxidative stress parameters in red blood cells after 3 months of deferasirox treatment.
New Insights into
Iron Overload Pathophysiology
“We are still learning about iron pathophysiology in MDS,” acknowledged Prof. de Witte. “For example, we now know that there is an increased level of non-transferrin-bound iron after every transfusion.” This is caused by transfusion of aged erythrocytes, he continued, a third of which are broken down within a week of transfusion, causing a spike in NTBI.
LIC has been little used as a measure of iron burden in MDS patients because of the risks inherent in liver biopsy. However, the increasing availability of MRI means LIC is becoming a more practical way of guiding the measurement of iron overload in MDS.
Prof. Gattermann presented a pooled analysis of LIC data from 124 iron-overloaded MDS patients in 4 different trials of deferasirox. The majority had severely elevated LIC: 56% had LIC >15 mg/g dw, highlighting that iron overload is a clinically relevant problem in MDS. These patients also had elevated ALT, which may indicate liver dysfunction. Treatment with deferasirox for 1 year reduced both LIC and serum ferritin. It also reduced ALT, which may be a marker of improved liver function. There was a significant correlation between serum ferritin, LIC and ALT.
Continuing advances in scientific knowledge, in conjunction with forthcoming prospective data from the TELESTO study and the EU MDS registry, should help clarify the optimal approach to managing iron overload in MDS.