Reports
Management of Axial Disease in Patients with PsA and SpA Pandemic
This report is based on medical evidence presented at sanctioned medical congress, from peer reviewed literature or opinion provided by a qualified healthcare practitioner. The consumption of the information contained within this report is intended for qualified Canadian healthcare practitioners only.
MEDICAL FRONTIERS - Annual Meeting of the American College of Rheumatology (ACR)
Atlanta, Georgia / November 8-13, 2019
Atlanta – The spine was in the spotlight at the ACR meeting in Atlanta. Axial psoriatic arthritis (PsA) and axial spondyloarthitis (SpA) featured in more than 200 posters and sessions, including landmark studies in neglected areas. Delegates saw results for PREVENT, the largest ever trial of a biologic in non-radiographic axial SpA (nr-axSpA), and MAXIMISE, the first prospective randomized clinical trial (RCT) to focus on axial manifestations of PsA. Two registration trials for IL-17 inhibitors (IL-17i) (secukinumab and ixekizumab) promised the first new drug class for nr-axSpA since TNF inhibitors. The first clinical trial data showing earlier treatment leads to better outcomes in SpA lit a fire under a host of sessions on earlier diagnosis and treatment in axial disease. “Researchers also showcased the largest real-life study of an IL-17i (secukinumab) in SpA and a host of patient-reported-outcome studies on the devastation caused by axial symptoms on patients’ lives.”
Chief Medical Editor: Dr. Léna Coïc, Montréal, Quebec
Clinicians often underestimate the significance of axial symptoms, said Dr. Atul Deodhar, Oregon Health and Science University, at the ACR conference: “Patients start with these axial symptoms, with back pain, and that’s what bothers the patient more – way more – than peripheral arthritis.” Dr. Xenophon Baraliakos, Rheumatology Center, Ruhr-University Bochum, Germany, said “Historically, we come from a place where psoriatic arthritis was seen as a different rheumatoid arthritis…we have not really paid much attention to the back.”
Dr. Philip Mease, University of Washington School of Medicine, said waking in the night was a “definitive” sign of inflammatory back pain (IBP) in SpA. The resultant day-time fatigue profoundly disrupts all aspects of patients’ lives – often in the crucial years of young adulthood. Dr. Mease said that two of his patients committed suicide because of the life disruption caused by their axial SpA. In the case of PsA, a poster led by Dr. Jessica Walsh, University of Utah, calculated that the presence of sacroiliitis on general health status measured by EQ-5D VAS was equivalent to having 24 other joints affected.
Diagnostic Challenges of Axial Symptoms
Despite the devastating impact of axial symptoms, referral and diagnosis may take a decade or more, largely because back pain is so common. “Once they get to us we know how to treat them – the big problem is getting them to us,” said Dr. Mease. “Patients labelled as chronic-back-pain patients are often shown to have inflammatory ankylosing spondylitis…it’s right under our nose; these patients are there.” In support of Dr. Mease’s remarks, the Canadian SASPIC cohort study showed that 47.6% of back-pain patients undergoing routine clinical evaluation for psoriasis, acute anterior uveitis or colitis had unsuspected axial SpA (N=246).
Several presentations focused on earlier recognition and diagnosis of axial symptoms. For example, a team led by Fabian Proft, Charité Universitätsmedizin, Berlin, devised an online self-referral tool in 181 SpA patients. The following parameters predicted axial SpA: chronic back pain (≥ 3 months) + back-pain onset before 45 years of age + ≥ two IBP parameters and ≥ one other SpA feature. The researchers plan to validate the tool in clinical practice.
Diagnostic challenges are particularly acute in non-radiographic axial SpA (nr-axSpA). The Spanish Atlas study found that nr-axSpA patients had longer diagnostic delays (10.1 versus 8.4 years) and lower biologic-therapy use than the r-axSpA patients (20.0% versus 36.7%; N=680).
Earlier Treatment Means Better Outcomes
in Axial Disease
Diagnostic delay of axial disease has serious clinical implications. At the conference, data from the C-axSpAnd Trial showed that delaying biologic treatment of nr-axSpA beyond 5 years resulted in significantly poorer outcomes. The study treated nr-axSpA patients with certolizumab, who were stratified by symptom duration (N=317). Patients with < 5 years of symptoms at baseline responded to the drug better than those with ≥ 5 years of symptoms on almost all pre-specified endpoints. These included ASDAS-MI, ASAS40, BASDAI, pain, fatigue, stiffness and SF-36 Physical. Presenter Dr. Jonathan Kay, University of Massachusetts, said, “These results imply that early diagnosis, enabling earlier treatment, is important for patients with nr-axSpA.”
Similarly, speakers recommended that PsA should be treated more aggressively. In her opening remarks, keynote speaker Dr. Susan Manzi, Allegheny Health Network, USA, said, “The concepts this year for psoriatic arthritis I think are clear: there’s evidence that we can be more aggressive with biologic therapy first line; RA has been doing that well before psoriasis.”
IL-17 Therapies Challenge Pre-Eminence
of TNFi’s
TNF-inhibitors are often considered to be first-line biologics for PsA and SpA. However, several presentations at the meeting called into question the real-life efficacy of TNFi’s in PsA and SpA. For example, the EuroSpA Research Collaboration followed a cohort of 16,230 patients in 15 European registries and found that only one-half of patients achieved patient-reported-outcome remission after 24 months of TNFi treatment. Dr. Bon San Koo, University of Seoul, South Korea, retrospectively analyzed ASDAS and BASDAI in 1,058 r-SpA patients treated with TNFi’s for a mean of 10.2 years. Dr. Bon’s team found residual high disease activity, measured by ASDAS-CRP, in 46.5% of the patients, despite adequate BASDAI scores. Dr. Bon said, “We were surprised when we found so many patients treated with TNFi’s, which are supposed to treat inflammation, with such a high inflammatory status and radiographic progression. [Now in our clinic] we don’t just ask about symptoms; we check for inflammation.”
With this background of TNFi inadequacy, new therapies such as IL-17i’s and IL-23i’s drew attention at the meeting and featured in Dr. Manzi’s keynote address. Referring to PsA, Dr. Manzi said, “IL-23 and IL-17 are key pathway targets. They’ve been proven to be superior to some of the TNFs.”
There were 41 abstracts on the IL-23i’s, and 123 abstracts on the IL-17i’s, at the meeting. One of the IL-17i studies broke ground as the first head-to-head comparison of biologics in PsA, demonstrating that an IL-17i was, indeed, superior to a TNFi. SPIRIT-H2H, presented by Prof. Josef Smolen, Medical University of Vienna, showed superiority for the humanized IL-17i ixekizumab against adalimumab for the primary endpoint of simultaneous ACR50 and PASI 100 through Week 52 (N=566; P<0.001). Five of the six PsA domains were covered by SPIRIT-H2H: peripheral arthritis, enthesitis, dactylitis, plaque PsO and nail PsO; axial disease data were not available.
In another IL-17i landmark moment, Dr. Baraliakos presented results for MAXIMISE, the first prospective RCT to focus on axial manifestations in PsA. Afterwards, an audience member congratulated Dr. Baraliakos, saying, “Obviously, this is something we needed for a long time.” The 52-week study of secukinumab, a fully human IL-17i, randomized 498 patients to 150 mg/300 mg secukinumab or placebo. After primary endpoint analysis at Week 12, placebo patients were crossed over into the secukinumab groups. Both doses of secukinumab significantly improved ASAS20 at Week 12 (primary/secondary endpoint for 300/150 mg dose), regardless of methotrexate use (P<0.001). Dr. Baraliakos stressed the significant reduction in spinal pain scores (see Figure 1): “[This] is important information…exactly this symptom that would bring them to the physician improved significantly as compared to placebo.” Delegates interested in axial SpA also saw pivotal IL-17i studies at the conference. Dr. Deodhar presented 52-week data for competing Phase-3 registration trials in nr-axSpA: PREVENT and COAST-X.PREVENT, a study of secukinumab 150 mg, was the largest-ever biologic trial in nr-axSpA, involving 555 patients for 52 weeks (primary endpoint) with a further 52-week extension. More patients on secukinumab achieved ASAS40 response versus placebo at Week 16 (41.5%/42.2% load/no load vs 29.2%; P<0.01). Secukinumab also improved BASDAI and BASDAI50 response, BASFI, physical function scores and quality of life (P<0.05 for all). Both sacroiliac-joint edema and high-sensitivity CRP fell significantly by Week 16 (P<0.05). Late-breaking results for the primary endpoint at 52 weeks, presented by Dr. Deodhar at the conference, confirmed that the ASAS40 response was maintained out to 1 year (see Figure 2).
Figure 1.
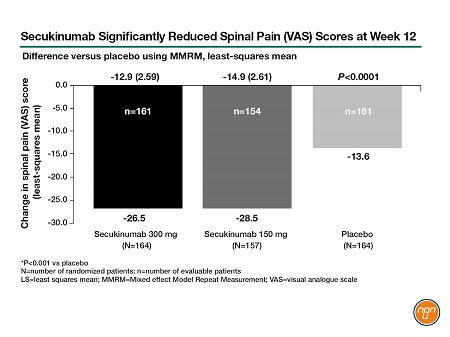
Figure 2.
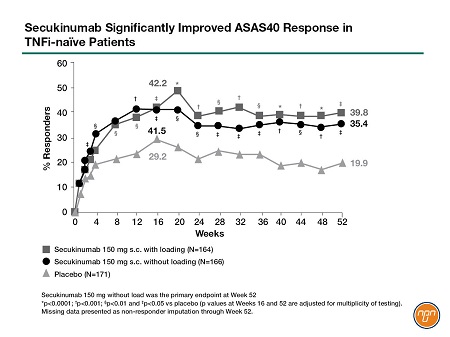
COAST-X tested ixekizumab (Q2W/Q4W) in 303 patients for 52 weeks. Significantly more patients on the ixekizumab regimens achieved ASAS40 response versus placebo at Weeks 16 and 52 (30%/31% vs 13%; P<0.01). It also significantly improved all major secondary endpoints, including sacroiliac-joint inflammation.
IL-17i’s in Real Life
The EuroSpa Research Collaboration Network presented data from the largest real-life study to date of an IL-17i therapy for axial SpA – secukinumab in 941 patients across 15 registries. Most patients had long disease duration and were treated with two or more previous DMARDs. At 12 months, 72.9% of the patients were still on secukinumab. The retention rate was highest (85.0%) in DMARD-naïve patients, who also responded to secukinumab best: 80% of the naïve patients had a BASDAI <4 at 12 months.
Conclusions
“These are clearly exciting times in rheumatology and immunology,” said ACR keynote speaker, Dr. Manzi. Nowhere was this more evident than in the science presented at the meeting on axial PsA and axial SpA. Clinicians and researchers called for faster and better recognition of axial disease, proposed innovative approaches to earlier diagnosis and shared landmark trials in new indications for promising therapies. The IL-17i’s, the first new drug class in nr-axSpA since the TNFi’s, have now accumulated the necessary data to revolutionize the treatment of radiographic and non-radiographic axial disease and transform the lives of PsA and SpA patients.
Questions and Answers
Questions and answers about the MAXIMISE and PREVENT studies with Dr. Xenofon Baraliakos, Senior Consultant, Rheumatology Centre, Ruhr-University Bochum, Germany, and Dr. Atul Deodhar, Professor of Medicine, Oregon Health and Science University, USA.
Q: What was unique about the MAXIMISE trial of the IL-17i secukinumab in PsA?
Dr. Xenofon Baraliakos: There have been trials that have looked retrospectively at the axial manifestations, but this is the very first placebo-controlled, randomized trial with a biologic prospectively performed to find out exactly those questions.
Q: Why the focus on axial manifestations?
Dr. Xenofon Baraliakos: Psoriatic arthritis is considered a peripheral disease – the small fingers, the knees, the small joints, maybe the large joints – we have not really paid much attention to the back. And we do know that these patients may get
a similar pattern over time as the ones that have ankylosing spondylitis. So it was an unclear clinical picture – inflammatory back pain in patients with peripheral arthritis.
Q: What surprised you about the results of MAXIMISE?
Dr. Xenofon Baraliakos: I thought that they would improve but the difference from placebo for these patients is so clear I have no doubt that we have done the right thing, we have identified the right patients and we have a very good clinical outcome.
So my big surprise was the clear message that we got, that the data was so robust: I didn’t think that we would have such good data.
Q: How does MAXIMISE change how we view psoriatic arthritis?
Dr. Xenofon Baraliakos: Historically, we come from a place where psoriatic arthritis was seen as a different rheumatoid arthritis…because of a lack of understanding of this disease. And now we see that psoriatic arthritis is indeed multi-faceted. [This] does not only mean musculoskeletal symptoms and other symptoms, it also means peripheral and axial symptoms together.
Q: What’s the implication of MAXIMISE for practice?
Dr. Xenofon Baraliakos: From the scientific point of view, we need to understand exactly what axial PsA is; from the clinical perspective, for the clinician every day, it is that the drugs are working, not only in the peripheral but also in the axial manifestations.
Q: Secukinumab is already approved for ankylosing spondylitis. Why was PREVENT needed?
Dr. Atul Deodhar: [Along with the ixekizumab trial] for the first time we’ve looked at a different mode of action – an IL-17 inhibitor – for non-radiographic axial SpA.
Q: What are the clinical implications of the PREVENT results?
Dr. Atul Deodhar: In the USA, only one drug is approved, even now, for non-radiographic axial SpA, and that is certolizumab. And now we’ve got two other drugs, ixekizumab and secukinumab. Both have completed 52-week, placebo-controlled studies, which is what the FDA mandated to do to see if this is a self-limiting disease. And the answer is, A, it’s not a self-limiting disease and B, it’s a second mode of action which works in this disease.
Based on scientific presentations made during the officially recognized sessions of the American College of Rheumatology (ACR) annual meeting in Atlanta Georgia, November 8-13, 2019