Reports
Maturing Clinical Experience with SGLT2 Inhibitors Is Clarifying Clinical Applications
This report is based on medical evidence presented at sanctioned medical congress, from peer reviewed literature or opinion provided by a qualified healthcare practitioner. The consumption of the information contained within this report is intended for qualified Canadian healthcare practitioners only.
ABSTRACTS in PERSPECTIVE - Based on presentations from the 73rd Scientific Sessions of the American Diabetes Association (ADA)
Chicago, Illinois / June 21-25, 2013
EDITORIAL OVERVIEW:
Philip A. McFarlane, MD, PhD, FRCPC
Division of Nephrology
St. Michael’s Hospital
University of Toronto
Toronto, Ontario
Jean-Francois Yale, MD, FRCPC
McGill University Health Centre
McGill University
Montreal, Quebec
The cumulative experience with inhibitors of the sodium-glucose transporter 2 (SGLT2) protein, updated at the 2013 ADA, outline broad potential applications in the control of diabetes mellitus. By blocking glucose reuptake in the kidneys and therefore inducing glycosuria, these oral agents exert an antidiabetic effect independent of insulin or ß-cell activity. They have been associated with a low risk of causing or exacerbating hypoglycemia, they produce a moderate weight loss, and they lower blood pressure (BP). Regulatory approval outside of Canada has now been granted to 2 agents: canagliflozin in the United States and dapagliflozin in the European Union. At the 2013 ADA, clinical data on these and other SGLT2 inhibitors, including a lengthening safety experience, has been generated largely in patients at early stages of type 2 diabetes mellitus (T2DM) but adjunctive use is also being explored in patients with difficult to control hyperglycemia, including those who are insulin-dependent. The potential for cardiovascular (CV) benefit independent of glycemic control is also being explored.
The SGLT2 Mechanism: Clinical Implications
In the kidney, sodium-glucose transporter proteins play a pivotal role in the homeostasis of glucose. Of several SGLT systems involved in human metabolism, such as SGLT1, which is present in the gut and the kidney, SGLT2 is the single most important mediator of renal glucose reabsorption (Ferrannini et al. Nat Rev Endocrinol 2012;8:495-502). It is present in the proximal tubules of the kidney and accounts for about 90% of this reabsorption (Misra J. Pharma Pharmacol 2013;65:317-27). By blocking the ability of SGLT2 to reabsorb glucose from the urinary filtrate, SGLT2 inhibitors cause glucose to be spilled in the final urine, but SGLT2 activity and the effect of SGLT2 inhibitors diminish as blood levels approach normal. The reduction in weight, which may be secondary to glucose excretion, and the reduction in systolic BP, which is attributed to increased sodium excretion, are additional potentially meaningful clinical benefits (Chao et al. Nat Rev Drug Discov 2010;9:551-9).
Unlike many antidiabetic agents, SGLT2 inhibitors have no effect on ß-cell function or insulin activity. As a result, there appears to be negligible risk of hypoglycemia (Chao et al. Nat Rev Drug Discov 2010;9:551-9). Overall, the tolerability profiles of SGLT2 inhibitors, with the exception of genital fungal and possibly urinary tract infections, have been comparable to a placebo, judging from Phase III clinical data presented at the 2013 ADA and previously. In data at the ADA, several studies specifically designed to evaluate the relative risk of genital and urinary tract infections (UTIs) found only modest elevations relative to placebo. There are potential risks of hypovolemia and azotemia, but these have been reported uncommonly to date (Nair et al. J Clin Endocrinol Metab 2010;95:34-42).
At the 2013 ADA, these features, initially established in the Phase III trials with canagliflozin and dapagliflozin, were reinforced by a large body of new data which expand the evidence of safety and tolerability and provide new information about how these agents may be best applied in clinical practice. In the expanding number of agents within this class to reach late stages of clinical testing, evidence is accumulating that at least some of the most attractive features are class effects. As yet, there is no consistent evidence of any serious adverse events from SGLT2 inhibition.
Renal Safety: SGLT2 Inhibitors in the Presence of CKD
Of new data presented at the ADA, the safety of SGLT2 inhibitors in T2DM patients with chronic kidney disease (CKD) was among the most significant. While SGLT2 inhibitors are not recommended in patients with severe renal impairment, this caution has been based on reduced efficacy rather than evidence of adverse effect on renal function. In the presence of a low estimated glomerular filtration rate (eGFR), glucose reabsorption is diminished, limiting the antidiabetic effect of SGLT2 inhibition. There has been no theoretical expectation of an adverse effect from SGLT2 inhibition on renal function, but extended follow-up was awaited for clinical confirmation. Several sets of new data were reassuring.
The most extensive data was generated by pooled Phase III late-breaker data with canagliflozin (Woo et al. ADA 2013: Abs 73-LB). The 1085 patients with CKD, defined as eGFR ≥30 but <60 mL/min/1.73 m2, were stratified into 2 groups. Those with eGFR ≥30 but <45 mL/min/1.73 m2 were compared to those ≥45 but <60 mL/min/1.73 m2. The reductions in hemoglobin A1c (HbA1c) at 26 weeks relative to placebo ranged from -0.23% in those with the greatest renal impairment taking the lowest dose of canagliflozin (100 mg) to -0.52% in those with better renal function taking the highest dose of canagliflozin (300 mg). Whether patients had low or moderate renal impairment, the advantage of canagliflozin on either dose relative to placebo was highly statistically significant (P<0.001). The relative systolic BP reductions on SGLT2 inhibition ranged from -1.8 to -4.9 mmHg. Adverse events of any kind leading to discontinuation, including those considered renal-related, were low and independent of baseline eGFR. Volume-related side effects were more frequent with canagliflozin than placebo in patients with low eGFR, particularly those on loop diuretics.
A second randomized but longer study produced similar results (Yale et al. ADA 2013; Abs 1075-P). In this 52-week study, patients with CKD, defined as ≥30 but <50 mL/min/1.73 m2, were randomized to one of 2 doses of canagliflozin or placebo. Both doses were significantly more effective than placebo for measures of glycemic control, and each reduced body weight and systolic BP significantly from baseline. Differences in renal function at the end of the follow-up were only modest. Specifically, eGFR relative to baseline was reduced 3% in the placebo group, 4% in the group on 100 mg canagliflozin and 8% in the group on 300 mg canagliflozin. At 52 weeks, blood urea nitrogen (BUN) on placebo, the lower dose of canagliflozin, and the higher dose of canagliflozin was increased 5%, 12%, and 16%, respectively.
This experience is so far consistent across SGLT2 inhibitors. In a study with empagliflozin in patients with T2DM and renal impairment subdivided into mild (≥60 but <90 mL/min/1.73 m2) and moderate (≥30 but <60 mL/min/1.73 m2), the reductions at 24 weeks from baseline in HbA1c and fasting plasma glucose were achieved on the SGLT2 inhibitor in both groups relative to placebo, but the reduction was less marked in the presence of moderate renal failure. Again, the SGLT2 inhibitor was well tolerated with no significant renal-related adverse events reported.
Genital Infections: Clarification of Risk
Large pooled data sets at the ADA were also useful for clarifying the risk of genital infection in patients taking a SGLT2 inhibitor. While it has long been understood that the increased urinary excretion of glucose, which is characteristic of SGLT2 inhibition, increases the rate of fungal infections, the pooled data indicate a low risk of UTI, which are potentially more serious. In a representative pooled analysis from 2477 patients participating in Phase III empagliflozin trials, the rates of UTI were not statistically different (8.2%, 9.3%, and 7.5%) in the placebo, lower dose, and higher dose groups, respectively (Kim et al. ADA 2013; Abs 74-LB). Similar data on UTI presented with canagliflozin (Nicolle et al. ADA 2013; Abs 1139-P) suggest that most genital infections are readily reversed with topical therapies and potentially circumvented with rigorous hygiene.
Clinical Role: Phase III Data
Phase III development programs with SGLT2 inhibitors have typically included multiple placebo-controlled trials in which these agents have been tested alone or in combination with one or more additional oral antidiabetic agents. The consistency of benefit across a large number of efficacy trials presented at the most recent ADA reassure initial evidence of the versatility of agents within this class.
Typical of monotherapy studies, a Phase III study of empagliflozin produced reductions in HbA1c ranging from 0.66% on a 10 mg dose to 0.78% on a 25 mg dose (Roden et al. ADA 2013; Abs 1085P). Reductions with both doses were significantly greater than with placebo (P<0.001), which did not reduce HbA1c at the end of 24 weeks, and similar to the 0.66% reduction in HbA1c achieved on the DPP-4 inhibitor sitagliptin. Weight loss of 2.26 kg and 2.48 kg respectively on the lower and higher doses of the SGLT2 inhibitor contrasted with a 0.5 kg weight gain on sitagliptin. Similarly, the 2.9 mmHg and 3.7 mmHg reductions in systolic BP in the low and high dose SGLT2 inhibitor, respectively, contrasted with a 0.5 mmHg increase on sitagliptin.
When SGLT2 inhibitors are combined with other antidiabetic agents, the reductions in HbA1c, body weight, and systolic blood pressure are similar to those observed on monotherapy but provided on top of the glycemic control provided by other active agents. In a study of dapagliflozin, patients on stable doses of metformin plus sitagliptin or metformin plus a sulfonylurea were randomized to receive the SGLT2 inhibitor or placebo. When evaluated at 24 weeks, there were no substantial changes in glycemic control in the group that received placebo. In those receiving dapagliflozin, HbA1c fell on average by 0.5% while body weight was reduced by approximately 2.0 kg (Jabbour et al. ADA 2013; Abs 1176-P).
The relative benefits of SGLT2 inhibitors continue to be observed across a variety of stratifications, including one comparing response to those 65 years or older and those younger (Bode et al. ADA 2013; Abs 76-LB). In this pooled analysis of patients participating in Phase III trials of canagliflozin, reductions in HbA1c were on average greater in the 1868 younger patients than in the 445 patients aged 65 years of age or older. Although older patients had a lower average baseline eGFR, which would be expected to reduce the glucose lowering effects of canagliflozin, the reductions in HbA1c in both groups relative to placebo were statistically significant. Moreover, the reductions in body weight and systolic BP on canagliflozin relative to placebo were commensurate with those observed in younger individuals. Genital infections occurred at a higher frequency in older patients, but serious adverse events in this population remained uncommon.
While the focus of the clinical trials with SGLT2 inhibitors remains on early use in T2DM, either alone or as a substitute for sulfonylurea as an adjunct to metformin, there is a theoretical role for these agents to improve control of blood glucose even in patients with insulin-dependent diabetes. Of several preliminary studies exploring this application, a single-arm clinical trial with empagliflozin provided representative and encouraging results (Perkins et al. ADA 2013; Abs 1074-P). In this 8-week study of 40 patients, the addition of the SGLT2 inhibitor to insulin was associated with a 0.4% reduction in HbA1c and an average reduction in symptomatic hypoglycemic events from 0.12/day to 0.04/day (P=0.0047). The average daily insulin dose declined from 55 to 46 U/day. Body weight was reduced on average by 2.7 kg.
The most recent studies expand evidence that SGLT2 inhibitors are effective, well tolerated, and impose a low risk of serious adverse events. Their role in the algorithm of oral therapies is still being explored, but their benefits are strongly juxtaposed to the limitations of sulfonylureas, which accelerate ß-cell exhaustion, induce weight gain, and impose a modest but significant risk of hypoglycemia.
SGLT2 Inhibitors and Cardiovascular Risk
The relevance of the favourable effects of SGLT2 inhibitors on CV risk factors is an intensifying focus of clinical study. In a summary of CV risk factor effects drawn from pooled Phase III empagliflozin data, reductions in HbA1c, systolic BP, diastolic BP, body weight, and uric acid were characterized as statistically significant and potentially clinically meaningful (Hach et al. ADA 2013; Abs 69-LB). Triglyceride levels were modestly reduced and HDL was modestly increased. The increases in total and LDL cholesterol with SGLT2 inhibitors is a source of concern, particularly for canagliflozin. Fortunately, several large CV trials with SGLT2 inhibitors are now testing the hypothesis that these agents are safe or could even offer CV protection. The CANagliflozin cardioVascular Assessment Study (CANVAS) is one example. Launched in 2009, CANVAS compares canagliflozin to placebo for a primary composite end point of major adverse CV events.
Conclusion
A large body of data from the 2013 ADA reinforces the promise of SGLT2 inhibitors for the control of diabetes mellitus. The lengthening follow-up from the first agents in this drug class to complete Phase III trials indicates sustained antidiabetic effect and a high degree of tolerability. Inhibition of glucose reabsorption in the kidney appears to be achieved with a very low risk of causing or exacerbating renal impairment. In the context of features that include a modest but sustained reduction in body weight, a low risk of hypoglycemia, and a BP lowering effect, it is reasonable to conclude that SGLT2 inhibitors will represent a meaningful addition to pharmacologic options in glycemic control.
ABSTRACT 69-LB
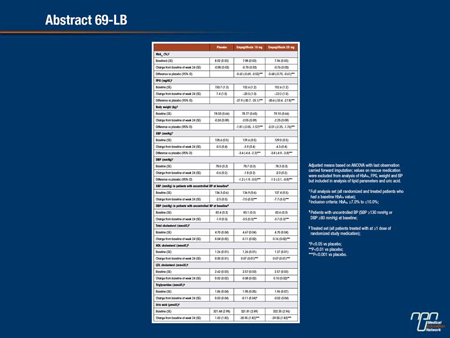
Empagliflozin Improves Glycemic Parameters and Cardiovascular Risk Factors in Patients with Type 2 Diabetes (T2DM): Pooled Data from Four Pivotal Phase III Trials
Thomas Hach, John Gerich, Afshin Salsali, Gabriel Kim, Stefan Hantel, Hans J. Woerle, Uli C. Broedl
We analyzed pooled data from 2477 patients with T2DM (mean [SD] age 55.6 [10.2] years, HbA1c 7.99 [0.85], BMI 28.7 [5.5]) from four randomized, placebo-controlled Phase III trials that investigated empagliflozin (EMPA) 10 mg or 25 mg given for 24 weeks as monotherapy, add-on to metformin (MET), add-on to MET + SU, or add-on to pioglitazone ± MET. Effects on HbA1c, fasting plasma glucose (FPG), weight, systolic and diastolic blood pressure (SBP and DBP) were evaluated in the full analysis set (placebo [PBO]: n=825, EMPA 10 mg: n=831, EMPA 25 mg: n=821). Effects on lipids and uric acid were evaluated in all treated patients (PBO: n=825, EMPA 10 mg:n=830, EMPA 25 mg: n=822). Effects on SBP and DBP were also evaluated in patients with uncontrolled BP (SBP ≥130 mmHg or DBP ≥80 mmHg) at baseline (PBO: n=501, EMPA 10 mg: n=517, EMPA 25 mg: n=506). EMPA significantly reduced HbA1c, FPG, weight, SBP, DBP and uric acid at week 24 vs PBO. Reductions in SBP and DBP were more pronounced in patients with uncontrolled BP at baseline. Small increases in HDL- and LDL-cholesterol and small decreases in triglyceride levels were observed with EMPA vs PBO. In conclusion, in a pooled analysis of data from four Phase III trials, 24 weeks’ treatment with EMPA 10 mg or 25 mg provided clinically meaningful improvements in glycemic parameters, weight, and BP, with positive effects on uric acid and small effects on lipids.
Commentary on abstract 69-LB
T2DM is typically accompanied by an array of related risk factors for cardiovascular disease, including obesity, hypertension, and dyslipidemias. These are likely to contribute to the high risk of cardiovascular death already imposed by macrovascular complications of diabetes. The ability of SGLT2 inhibitors to lower body weight and blood pressure have drawn attention to the potential for cardiovascular benefit independent of glycemic control. In this study, the focus on cardiovascular risk factors quantifies the effects on risk factors across a Phase III development program with empagliflozin. While these effects appear to be shared by other SGLT2 inhibitors, the data encourage efforts now underway to test the hypothesis that SGLT2 inhibitors can provide protection against cardiovascular events when compared to placebo. An antidiabetic agent capable of offering cardiovascular risk reduction across multiple risk factors would be considered a substantial advance in clinical management.
Questions and answers with John Gerich, MD
Q: The association between SGLT2 inhibitors and reductions in body weight and blood pressure has been reported repeatedly, but is there evidence they are clinically meaningful?
A: The reductions in both body weight and blood pressure are modest but they are sustained and they are of a magnitude that suggests a potential for impact on cardiovascular risk.
Q: Your data show a modest increase in LDL and total cholesterol but a modest reduction in triglycerides and an increase in HDL. When evaluated together, does this predict a neutral effect?
A: This would be a simplistic characterization. Dyslipidemia in T2DM typically includes hypertriglyceridemia and depressed HDL. The modest but favourable effects on these parameters relative to cardiovascular risk could potentially be more meaningful than the modest increases observed in LDL.
Q: Why was uric acid measured?
A: The predictive value of hyperuricemia for cardiovascular disease appears to be modest in otherwise healthy individuals, but it is a robust additive risk marker in individuals who have multiple cardiovascular risks. The substantial reduction in uric acid on SGLT2 inhibitors is intriguing.
Q: Do you feel a trial with cardiovascular events as an endpoint is warranted?
A: Cardiovascular disease is the single most important cause of death in patients with T2DM. The reductions in risk factors we observed in these pooled data are substantial and justify a trial to evaluate whether they are clinically meaningful.
Abstract 73-LB
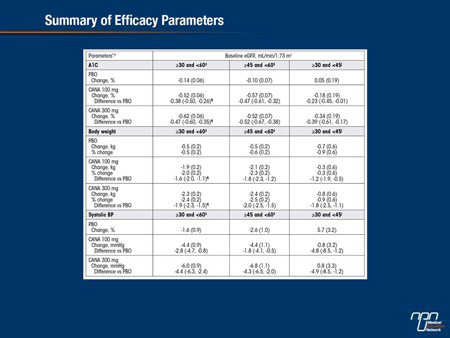
Canagliflozin (CANA) is Effective and Generally Well Tolerated in Subjects with Type 2 Diabetes Mellitus (T2DM) and Stage 3 Chronic Kidney Disease (CKD)
Vincent Woo, Melanie Davies, Dick De Zeeuw, George Bakris
The efficacy/safety of the SGLT2 inhibitor, CANA, was assessed by a pooled analysis in subjects with T2DM from 4 randomized, placebo (PBO)-controlled studies (Wk 18, 1 study; Wk 26, 3 studies) with an eGFR ≥30 and <60 mL/min/1.73 m2 (N = 1,085) and in subgroups with eGFR ≥45 and <60 (n = 721) or ≥30 and <45 mL/min/1.73 m2 (n = 364). CANA 100 and 300 mg reduced A1C, body weight, and systolic BP versus PBO across populations (Table); A1C and body weight changes were larger in subjects with eGFR ≥45 than <45 mL/min/1.73 m2 . For the pooled CANA group, overall AE rates were higher than PBO across populations (eGFR ≥30 to <60: 74.7% vs 70.4%; ≥45: 71.0% vs 66.9%; <45: 81.5% vs 78.4%); serious AE rates were higher with PBO than CANA and AE-related discontinuation rates were low across populations. Rates of osmotic diuresis-related AEs (eg, pollakiuria, polyuria) were higher with CANA than PBO in subjects with eGFR ≥30 and <60 (4.0% vs 3.7%) and <45 mL/min/1.73 m2 (4.8% vs 2.6%). Rates of AEs related to reduced intravascular volume (eg, postural dizziness, orthostatic hypotension) were higher with CANA than PBO across populations (eGFR ≥30 to <60: 6.8% vs 2.6%; ≥45: 5.9% vs 3.4%; <45: 8.9% vs 1.7%). Rates of renal-related AEs that were serious or led to discontinuation were low and similar across groups. In summary, in subjects with T2DM and Stage 3 CKD, CANA reduced A1C with a greater effect in subjects with higher eGFR, and was generally well tolerated.
Commentary on abstract 73-LB
The most significant limitation on the use of SGLT2 inhibitors in the treatment of diabetes is the presence of severe renal impairment. In such individuals, SGLT2 inhibition offers a more limited opportunity to prevent glucose reabsorption due to a lower rate of blood filtration. This analysis of patients with moderate to mild chronic kidney disease (CKD) provides an opportunity to evaluate how diminishing renal function affects SGLT2 efficacy and whether these agents are safe when renal function is impaired. As expected, there was less relative improvement in glycemic control achieved with the SGLT2 inhibitor canagliflozin in those with the greatest renal impairment, but HbA1c reductions in this group remained significant relative to placebo. Serious renal-related adverse events were not observed with canagliflozin therapy. Although canagliflozin, relative to placebo, was associated with an overall increase in adverse events related to intravascular volume, the rates of serious volume-related events were not higher. Canagliflozin did not appear to exacerbate existing CKD.
Questions and answers with Vincent Woo, MD
Q: You note that hypoglycemia was more common on canagliflozin than placebo when background medicine was used. Is this likely related to renal function or to the underlying risk from the medications with which canagliflozin was combined?
A: The risk was most likely related to the use of insulin and sulfonylureas.
Q: Glycosuria has been a traditional sign of uncontrolled diabetes, but this is the mechanism of benefit from SGLT2 inhibitors; is there a reason to be concerned that glycosuria may be harmful to the kidneys?
A: Glycosuria has not been used clinically as a sign of uncontrolled diabetes mellitus for 20 years. There were no concerns raised about the effects of glycosuria thus far.
Q: Was there decline in renal function observed in the CKD patients treated with canagliflozin in this analysis over time?
A: No significant change in renal function was observed.
Q: In labeling from the US FDA, canagliflozin is contraindicated when the eGFR is <45 mL/min/1.73 m2; do you think this is a reasonable threshold?
A: Yes. Even though antidiabetic effect was observed in those with eGFR below this level, the threshold is reasonable for general practice.
Abstracts 76-LB
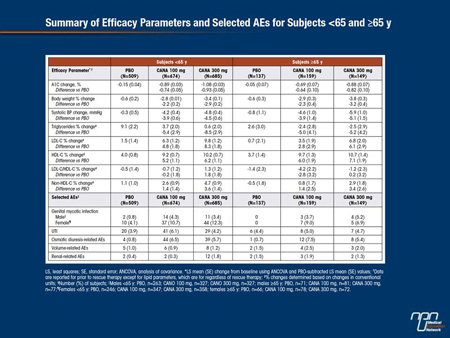
Efficacy and Safety of Canagliflozin in Older Subjects with Type 2 Diabetes Mellitus
Bruce W. Bode, Alan Sinclair, Stewart Harris, Ujjwala Vijapurkar, Cristiana Gassmann-Mayer, Albert Fung, Wayne Shaw, Keith Usiskin, Mehul Desai, Gary Meininger
The efficacy and safety of CANA, an SGLT2 inhibitor, were evaluated using pooled data in subjects with T2DM from 4 randomized, placebo (PBO)-controlled, 26-week studies (N = 2,313) and analyzed by age: <65 y (n = 1,868; male, 49.1%; mean age, 52.8 y; A1C, 8.0%; body weight, 90.1 kg; eGFR, 90.8 mL/min/1.73 m2) or ≥65 y (n = 445; male, 51.5%; mean age, 69.3 y; A1C, 7.9%; body weight, 85.1 kg; eGFR, 76.9 mL/min/1.73 m2). CANA 100 and 300 mg reduced A1C, body weight, and systolic BP relative to PBO in subjects <65 and ≥65 y (Table); similar lipid changes were seen in both age groups. Overall adverse event (AE) rates were similar with CANA 100 and 300 mg and PBO in subjects <65 y (59.9%, 59.0%, 58.9%) and ≥65 y (61.0%, 60.4%, 61.3%). Serious AE and AE-related discontinuation rates were similar with CANA 100 and 300 mg and PBO in subjects <65 y (serious AEs: 2.5%, 2.5%, 3.3%; AE-related discontinuations: 3.3%, 3.2%, 2.8%), and higher with CANA 100 mg than CANA 300 mg or PBO in subjects ≥65 y (serious AEs: 6.9%, 3.4%, 3.6%; AE-related discontinuations: 8.8%, 5.4%, 4.4%). As in subjects <65 y, those ≥65 y who received CANA had higher rates than PBO of genital mycotic infections in women and men and osmotic diuresis-related AEs; rates of AEs related to reduced intravascular volume were low in both age groups. UTI and renal-related AE rates were similar across groups in subjects ≥65 y. In summary, both CANA doses provided reductions in A1C and body weight and were generally well tolerated in older subjects with T2DM.
Commentary on abstract 76-LB
When new therapies with novel mechanisms of action are introduced, it is important to evaluate efficacy and safety across a broad spectrum of populations. Due to age-related changes in a variety of physiologic systems, older patients are a particularly appropriate focus of such analyses. In this study, pooled Phase III data with canagliflozin allowed efficacy and safety to be evaluated in a relatively large group of individuals over the age of 65. When older patients were compared to younger patients, the average HbA1c reductions were slightly lower in the older group. However, when the HbA1c reductions on canagliflozin were compared to placebo, the relative advantage for the SGLT2 inhibitor was highly significant in both older and younger patients. The lower average eGFR, a predictor of response to SGLT2 inhibitors, may explain the diminished average HbA1c reductions in older individuals. Reductions in body weight and systolic blood pressure on canagliflozin were of similar magnitude in older and younger individuals. Adverse events attributed to canagliflozin were more commonly observed in older individuals, but this agent remained well tolerated in both groups. The sum of the data suggests age should not be a major factor in considering use of this agent.
Questions and answers with Bruce W. Bode, MD
Q: Was there any theoretical concern that a SGLT2 inhibitor would be less effective in older individuals?
A: Renal function diminishes with age, so it is reasonable to predict that older patients may not respond as well to an agent that is dependent on renal function for its activity. The slightly lower average HbA1c reductions in older individuals were predicted on this basis.
Q: Yet, you conclude that canagliflozin remains effective in older individuals?
A: The average differences in HbA1c reductions were quite modest. On the lower dose the reductions in younger and older individuals were 0.89% and 0.69%, respectively. On the higher dose, the reductions from baseline were 1.08% and 0.88%. If lower, the average HbA1c reductions in older individuals remained substantial.
Q: What about safety?
A: The rates of genital infections were slightly higher in older individuals as were adverse events related to osmotic diuresis. However, the overall rates of adverse events thought to be related to canagliflozin were only very modestly elevated. Serious adverse events were more common in older individuals, but many of these were not considered to be drug related.
Q: Why were the reductions in body weight and systolic blood pressure at least as great in older patients relative to younger patients when these, too, would be expected to be renal dependent?
A: Older patients appear to be more responsive to the diuretic activity of SGLT2 inhibition. To the extent that weight loss and blood pressure reductions are clinically meaningful, older patients may derive more benefit from these actions than younger patients.
Abstract 238
CANA is an SGLT2 inhibitor in development for the treatment of T2DM. This randomized, double-blind study enrolled subjects with T2DM on MET (N = 1,284; mean age, 55.4 y; A1C, 7.9%; fasting plasma glucose [FPG], 169.1 mg/dL; BMI, 31.8 kg/m2) and had a 26-week, placebo (PBO)- and active-controlled period (CANA 100 and 300 mg, SITA 100 mg, PBO [2:2:2:1]) followed by a 26-week, active-controlled period (PBO-treated subjects switched to SITA [data not reported]). At Week 52, both CANA doses were non-inferior to SITA and CANA 300 mg was statistically superior to SITA in A1C-lowering. CANA significantly reduced FPG, body weight, and systolic BP relative to SITA (P<0.001 for all), with a numerical increase in HDL-C and a slight increase in LDL-C.
Overall AE rates were slightly higher with CANA 100 mg than CANA 300 mg and SITA (72%, 63%, 65%). Serious AE and AE-related discontinuation rates were low across groups. Rates of documented hypoglycemia (7% vs 4%), genital mycotic infections (women, 11% vs 2%; men, 4% vs 1%), and AEs related to osmotic diuresis (6% vs 2%) were higher with the pooled CANA group versus SITA. For the pooled CANA group and SITA, UTI rates were 6% for both and rates of AEs related to reduced intravascular volume (1% vs 2%) and renal function (3% for both) were low. In summary, CANA 300 mg improved glycemic control and both CANA doses reduced body weight relative to SITA, and were generally well tolerated in subjects with T2DM on MET over 52 weeks.

Commentary on abstract 238
Initial studies with SGLT2 inhibitors were placebo-controlled, but the increasing number of studies that compare these agents to another active therapy will help define where these agents can best be deployed in routine care. In this study of 1,284 T2DM patients taking metformin, the SGLT2 inhibitor canagliflozin was compared to the DPP-4 inhibitor sitagliptin in a 26-week study with a placebo arm. In a 26-week extension, patients initially randomized to placebo were placed on sitagliptin. The results reported here represent a comparison of canagliflozin and sitagliptin at the end of 52 weeks. Canagliflozin was found non-inferior to sitagliptin for the primary outcome of HbA1c reductions. Body weight, fasting blood glucose, and systolic blood pressure (all P<0.001) were significantly reduced on canagliflozin relative to sitagliptin. Serious adverse events were observed in low rates across all groups, although canagliflozin was associated with a higher rate of genital infections as well as adverse events associated with osmotic diuresis.
Questions and answers with Fernando J. Lavalle Gonzalez, MD
Q: What was the rationale for this comparison?
A: Both canagliflozin and sitagliptin are associated with substantial improvements in glycemic control with a favourable side effect profile. Each is attractive as an adjunctive agent to metformin, particularly in place of a sulfonylurea.
Q: Were there any surprises in this comparison?
A: The glucose lowering effects and the side effect profiles on both agents were consistent with the previous clinical experience with each agent. For HbA1c lowering, the data confirmed the study hypothesis that canagliflozin would be non-inferior to sitagliptin. It is notable, however, that average reductions in fasting plasma glucose were about twice as high on canagliflozin.
Q: Canagliflozin was associated with greater weight reductions and reductions in systolic blood pressure. Were these relative benefits consistent over the course of the study?
A: Blood pressure reductions on canagliflozin are generally observed within a few days of initiating therapy. Weight loss is observed over several weeks, but each was sustained over long-term therapy. The relative advantage for canagliflozin relative to sitagliptin was similar at 26 weeks and 52 weeks.
Q: What conclusions can be drawn about the relative utility of these two agents in T2DM patients on metformin?
A: The study provides evidence that canagliflozin is at least as effective as sitagliptin for glycemic control but provides additional favourable effects such as the reduction in body weight and systolic blood pressure. Other considerations may be important when selecting therapy for specific individuals.
Abstract 242
Plasma Glucose Reduction with the SGLT2 Inhibitor Dapagliflozin Improves Insulin Sensitivity and Insulin Secretion in T2DM Muhammad A. Abdul-Ghani, Aurora Merovci, Carolina Solis-Herrera, Roy Eldor, Divjit Tripathy, Giuseppe Daniele, Ralph A. Defronzo
Chronic hyperglycemia causes microvascular complications and aggravates the core defects (insulin resistance and beta cell dysfunction) present in T2DM. In diabetic animals lowering plasma glucose conc with phlorizin improves insulin sensitivity/secretion. In the present study we examined whether lowering plasma glucose by inhibiting SGLT2 improves insulin sensitivity/secretion in T2DM.
11 T2DM males (age= 51+3; BMI= 31.1+1.6; A1c=8.2+0.3%) received: (i) 75-gram OGTT and (ii) euglycemic insulin (+80 μU/m2/min) clamp. After completing baselines studies, subjects were admitted to the CRC for measurement of basal hepatic glucose production with 3H-glucose. After a 3-hour tracer equilibration, subjects ingested 10 mg dapagliflozin and 3H-glucose infusion was continued for 6 hours. Subjects then were discharged on dapagliflozin (10 mg/d) for 7 days. On days 6 and 7, OGTT and insulin clamp were repeated.
Datagliflozin increased urinary glucose excretion by 69+7 grams/24 h and this was accompanied by reductions in FPG (28+6 mg/dl) and 2-h plasma glucose (72+11 mg/dl) during OGTT (P<0.001 for both). The decrease in plasma glucose was accompanied by 28% increase (4.6+0.6 to 5.9+0.5 mg/kg.min, P<0.001) in insulin-stimulated total body glucose disposal (TGD) rate, which remained significant (P<0.01) after correcting for urinary glucose loss, documenting improved muscle sensitivity to insulin. Surprisingly, the decrease in plasma glucose was accompanied by a 16% (P<0.05) increase in HGP which correlated with a 23% increase in plasma glucagon conc. Dapagliflozin also caused a 2-fold increase in beta cell function (insulin secretion/insulin resistance or disposition index).
Conclusions: Lowering plasma glucose conc with the SGLT2 inhibitor, dapagliflozin, improves the two core defects (insulin resistance and beta cell dysfunction) present in T2DM. These results provide the first conclusive proof for the “glucotoxicity” hypothesis in vivo in man.
Commentary on abstract 242
Although the antidiabetic effect of SGLT2 inhibitors is achieved independent of ß-cell function and insulin activity, there are animal data to suggest that reductions in plasma glucose improve insulin sensitivity, which, as a consequence, may preserve ß-cell activity. This study in a small number of T2DM patients provides clinical evidence to support these effects. Conducted with dapagliflozin, the reductions in glucose with SGLT2 inhibition were accompanied by improved muscle sensitivity to insulin. As measured by the insulin secretion/insulin resistance index, dapagliflozin was associated with a 2-fold increase in ß-cell function. Moreover, there was a 23% increase in plasma glucagon concentration. The data overall support the hypothesis that hyperglycemia exerts toxic effects on ß-cell function that can be reversed with effective glucose lowering. They also suggest that SGLT2 inhibition exerts a favourable effect on the physiology of blood glucose homeostasis that may be relevant to slowing or changing the natural history of T2DM progression.
Questions and answers with Muhammad A. Abdul-Ghani, MD, PhD
Q: Does this study suggest that hyperglycemia directly causes insulin resistance?
A: There are already animal data that provide evidence that chronic hyperglycemia causes glucotoxicity that not only increases risk of microvascular complications but exacerbates the problems that lead to progressive diabetes, such as reduced insulin sensitivity.
Q: Why conduct this study with a SGLT2 inhibitor rather than another therapy associated with blood glucose reductions, such as metformin?
A: SGLT2 inhibitors are particularly effective for sustained and significant reductions achieved by filtering glucose from the blood rather than preventing new glucose production. They are a better tool for evaluating the concept of glucotoxicity.
Q: Does the improvement in ß-cell function suggest that the blood-glucose lowering effect of SGLT2 inhibitors have the potential to alter the natural history of T2DM?
A: This study was conducted with a small number of patients over a period of 8 days. It is more of a hypothesis-generating study than a proof of concept, but the potential that sustained reductions in blood glucose may protect the ß-cell is intriguing.
Q: What is the mechanism of the upregulation of glucagon?
A: This may be related to feedback signals from inhibition of SGLT2, but this effect was unexpected, and we are now only just looking at the potential mechanisms.
Abstract 1075-P
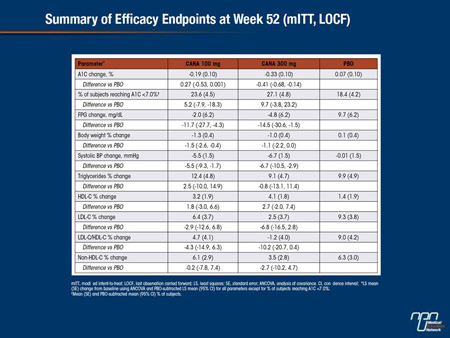
Efficacy and Safety of Canagliflozin in Subjects with Type 2 Diabetes Mellitus and Chronic Kidney Disease (CKD) over 52 Weeks
Jean-François Yale, George L. Bakris, Bertrand Cariou, Javier Nieto Iglesias, Ewa Wajs, Katherine Figueroa, Joel Jiang, Keith Usiskin, Gary Meininger
CANA is an SGLT2 inhibitor in development for the treatment of T2DM. This randomized, double-blind study enrolled subjects with T2DM and Stage 3 CKD (eGFR ≥30 and <50 mL/min/1.73 m2). Subjects (N = 269; mean age, 68.5 y; A1C, 8.0%; fasting plasma glucose [FPG], 164.0 mg/dL; BMI, 33.0 kg/m2; eGFR, 39 mL/min/1.73 m2; median albumin/creatinine ratio [ACR], 30 μg/mg) received CANA 100 or 300 mg or placebo [PBO] added to current therapy (74% on insulin) during a 26-week core period followed by a 26-week extension (n = 207). Over 52 weeks, CANA 100 and 300 mg reduced A1C, FPG, body weight, and systolic BP, with small increases in HDL-C and small decreases in LDL-C relative to PBO.
Overall AE rates were 86%, 81%, and 87% and serious AE rates were 20%, 24%, and 27% with CANA 100 and 300 mg and PBO; AE-related discontinuation rates were low across groups. Proportion of subjects with hypoglycemia (64%, 59%, 46%) and rates of osmotic diuresis-related AEs (eg, pollakiuria; <5% per group) were higher with CANA 100 and 300 mg than PBO; rates of UTIs and AEs related to reduced intravascular volume (eg, postural dizziness) were higher with CANA 300 mg than PBO. Increases in BUN (12%, 16%, 5%) and decreases in eGFR (-4%, -8%, -3%) and median ACR (-1, -7, 2 μg/mg) were seen with CANA 100 and 300 mg relative to PBO. In summary, CANA improved glycemic control and was generally well tolerated in subjects with T2DM and Stage 3 CKD over 52 weeks, similar to findings at Week 26.
Commentary on abstract 1075-P
In current regulatory guidelines for canagliflozin in the United States, where Food and Drug Administration (FDA) approval has been granted, moderate renal impairment defined as an eGFR <45 mL/min/1.73 m2 is listed as a relative contraindication. However, the long-term efficacy and safety in patients with varying degrees of renal impairment remain incompletely defined. In this study, the efficacy and safety of canagliflozin was evaluated in patients who entered Phase III trials with chronic kidney disease, defined as eGFR ≥30 but <50 mL/min/1.73 m2 . At the end of 52 weeks, both the 100 and 300 mg doses of canagliflozin significantly reduced HbA1c, fasting plasma glucose, body weight, and systolic blood pressure relative to baseline and placebo. The rates of serious adverse events in the 100 mg canagliflozin, 300 mg canagliflozin, and placebo groups were 20%, 24%, and 27%, respectively. Reductions in eGFR were observed in 4%, 8%, and 3%, respectively, while blood urea nitrogen (BUN) was increased by 12%, 15%, and 5%, respectively. Relative to baseline, changes at 52 weeks were similar to those observed at 26 weeks.
Questions and answers with Jean-François Yale, MD
Q: SGLT2 inhibitors reduce HBA1c by increasing glucose excretion in the urine. Traditionally, glycosuria has been considered evidence of uncontrolled diabetes. Are you concerned that physicians who place patients on a SGLT2 inhibitor will be concerned by glycosuria?
A: Only physicians older than I am will remember the days when measuring glucose in the urine was routinely employed to evaluate control of diabetes. I do not think there is much danger that physicians will interpret glycosuria as a sign that diabetes is uncontrolled or adversely affecting kidney function. Glycosuria can actually be presented as a defense mechanism that the body has to prevent negative effects of acute hyperglycemia, and SGLT2 inhibitors may enhance this protective mechanism.
Q: However, the attention to kidney function in patients taking SGLT2 inhibitors has produced several studies at this meeting. Is there a theoretical risk to the kidney?
A: The kidney is an important organ, so I think that attention would be paid to renal function with any new agent. In addition, SGLT2 inhibitors exert their antidiabetic effect in the kidney, so there is a reason to be interested in long-term safety. I am not aware that blocking glucose reabsorption would be predicted to induce any harm to the kidney, but these safety data are reassuring.
Q: Do the modest decreases in eGFR at 52 weeks in this study suggest that SGLT2 inhibition is accelerating renal impairment in patients with CKD?
A: I do not think we have any strong evidence yet that SGLT2 inhibitors produce any permanent alteration in renal function. There seems to be an acute reduction in eGFR, potentially related to reductions in circulating volume at drug initiation, followed by a gradual return towards baseline, while there is a slow but progressive decrease in placebo patients.
Q: Is there sufficient evidence to conclude that these agents are safe in regard to kidney function?
A: There is now a substantial body of evidence, including another analysis with canagliflozin presented at this meeting, that suggests a low risk of renal-related adverse events even in patients with chronic kidney disease. So far, there are no significant safety concerns.
Abstract 1085-P
Empagliflozin Monotherapy Improves Glucose Control in Drug-Naïve Patients with Type 2 Diabetes (T2DM)
Michael Roden, Jianping Weng, Jens Eilbracht, Bruno Delafont, Gabriel Kim, Hans J. Woerle,
Uli C. Broedl
A Phase III trial investigated the efficacy and safety of empagliflozin (EMPA) in drug-naïve patients with T2DM. Patients (mean age 55.0 years; mean BMI 28.4 kg/m2) were randomized double-blind and treated with EMPA 10 or 25 mg qd (n=224 each), sitagliptin 100 mg qd (SITA; n=223), or placebo (PBO; n=228) for 24 weeks. Patients with HbA1c >10% (n=87) received open-label EMPA 25 mg qd for 24 weeks. Primary endpoint was change from baseline in HbA1c at week 24. Key secondary endpoints were change from baseline in weight, systolic blood pressure (SBP) and diastolic BP (DBP) at week 24. EMPA significantly reduced HbA1c, weight, and SBP versus PBO. DBP changes were not significant versus PBO. Weight loss of >5% was achieved by 22.8% of patients on EMPA 10 mg, 29.0% on EMPA 25 mg, 6.3% on SITA and 4.4% on PBO. Proportion of randomized patients with adverse events (AEs) was similar with PBO (61.1%), EMPA (54.9-60.5%), and SITA (53.4%). Hypoglycemia (plasma glucose ≤70 mg/dL and/or requiring assistance) was reported by 1 (0.4%) patient per randomized group; none required assistance. AEs consistent with urinary tract infection were reported in 5.4-6.7% of randomized patients on EMPA, 5.2% on PBO and 4.9% on SITA. AEs consistent with genital infection were reported in 3.1-4.0% of randomized patients on EMPA, 0 on PBO and 0.9% on SITA.
To conclude, EMPA 10 mg and 25 mg for 24 weeks reduced HbA1c, weight, and SBP versus PBO, and were well tolerated in drug-naïve patients with T2DM.
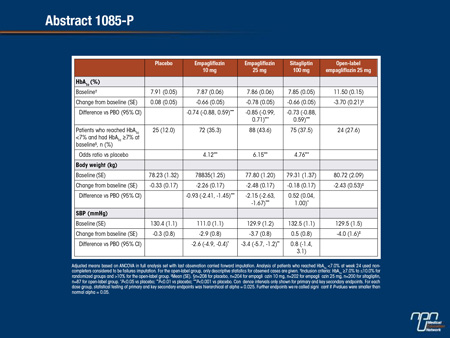
Commentary on abstract 1085-P
Metformin is widely acknowledged as first-line therapy in most patients with T2DM. SGLT2 inhibitors, which are also associated with a low risk of hypoglycemia and are well tolerated, may be an alternative. In this Phase III study, 999 drug-naïve patients were randomized to 10 mg empagliflozin, 25 mg empagliflozin, 100 mg of sitagliptin or placebo. Relative to baseline, HbA1c at the end of 24 weeks was unchanged on placebo, reduced by 0.66% on 10 mg empagliflozin, reduced by 0.78% on 25 mg empagliflozin, and reduced by 0.66% on sitagliptin. Body weight was significantly reduced from baseline on both doses of empagliflozin but unchanged on placebo and slightly increased on sitagliptin. Systolic blood pressure was essentially unchanged on both placebo and sitagliptin but reduced by approximately 3 mmHg on both doses of empagliflozin. Genital infections were more common on empagliflozin than placebo or sitagliptin, but serious adverse events were uncommon in all groups.
Questions and answers with Michael Roden, MD
Q: The data suggest that sitagliptin performed about the same as the lower dose of empagliflozin but provided somewhat less glycemic control than the higher dose.
A: The differences in the HbA1c reductions at 24 weeks were not significantly different between any of the active therapies, but the numerical advantage of the higher dose of empagliflozin was consistent for HbA1c and for the proportion of patients who reached HbA1c <7%.
Q: The trials consistently demonstrate an advantage for SGLT2 inhibitors over other antidiabetic drugs for reductions in body weight and systolic blood pressure, but what do these mean clinically?
A: In patients with T2DM, there are well documented benefits from tighter blood pressure control and from weight loss, but we cannot say from a study like this that improvements in these parameters will confer an advantage on clinical outcomes. We need studies designed to prove that these differences are meaningful, but weight loss and improved blood pressure control are desirable effects.
Q: Can any conclusions be drawn about empagliflozin in first-line therapy from these results?
A: The main conclusion is that empagliflozin is effective and well tolerated for reducing HbA1c in drug-naïve patients relative to placebo. Based on this and other studies, SGLT2 inhibitors show promise as first-line agents, but this study was not specifically designed to test their role relative to other options.
Abstract 1098-P
Efficacy and Safety of Canagliflozin (CANA) in Subjects With Type 2 Diabetes Mellitus (T2DM) on Metformin (MET) and Pioglitazone (PIO) Over 52 Weeks
Thomas Forst, Robert Guthrie, Ronald Goldenberg, Jacqueline Yee, Ujjwala Vijapurkar, Gary Meininger, Peter Stein
CANA is an SGLT2 inhibitor in development for the treatment of T2DM. This randomized, double-blind study enrolled subjects with T2DM on MET and PIO (N = 342) and included a 26-week core period (CANA 100 and 300 mg vs placebo [PBO]) and a 26-week extension period (n = 275; mean age, 56.9 y; A1C, 7.9%; fasting plasma glucose [FPG], 162.2 mg/dL; BMI, 32.5 kg/m2; PBO group switched to sitagliptin 100 mg [PBO/SITA]). Efficacy data at 52 weeks are reported for CANA 100 and 300 mg (SITA used to maintain double-blind and as a control group, not as efficacy comparator); safety data are shown for both CANA doses and PBO/SITA. At Week 52, CANA 100 and 300 mg lowered A1C and FPG from baseline with a substantial proportion of subjects reaching A1C <7.0%; reductions in body weight and systolic BP, and increases in HDL-C, LDL-C, and triglycerides were also seen.
Overall AE rates were lower with CANA 100 mg than CANA 300 mg and PBO/SITA (70%, 76%, 77%). Rates of serious AEs, AE-related discontinuations, UTIs, and hypoglycemia were similar across groups. Higher rates of AEs related to osmotic diuresis (ie, thirst, pollakiuria; <7% per AE), reduced intravascular volume (ie, postural dizziness, orthostatic hypotension; <2% per AE), and genital mycotic infections were seen in the pooled CANA group versus PBO/SITA. In summary, CANA improved glycemic control, reduced body weight, and was generally well tolerated in subjects with T2DM on MET and PIO over 52 weeks.

Commentary on abstract 1098-P
In this study, the efficacy and safety of adding canagliflozin in either a 100 mg or 300 mg dose to a combination of metformin and the thiazolidinedione pioglitazone was tested in a placebo-controlled 26-week trial followed by a 26-week extension in which placebo patients were switched to sitagliptin. At week 52, the reduction in HbA1c from baseline was 0.98% on the 100 mg dose and 1.07% on the 300 mg dose. The proportion of patients achieving HbA1c <7% was 52.9% and 64.6% on the low and high doses, respectively. The reductions in body weight were 2.9% and 4%, respectively, and the reductions in systolic blood pressure were 3.5 mmHg and 4.3 mmHg, respectively. The rates of serious adverse events, discontinuations due to adverse events, and rates of hypoglycemia were low and similar across study arms. Rates of osmotic diuresis and genital mycotic infections were higher in the pooled canagliflozin groups relative to placebo, but the authors concluded that this combination of metformin, canagliflozin and pioglitazone was well tolerated overall.
Questions and answers with Robert Guthrie, MD
Q: Why did you look at this combination?
A: Most T2DM patients require 2 or more antidiabetic drugs. The introduction of SGLT2 inhibitors is going to change treatment algorithms. I expect SGLT2 inhibitors to largely replace sulfonylureas which have numerous disadvantages. In a patient that requires three drugs, these specific agents represent a potentially attractive combination.
Q: If patients start on metformin, which comes second?
A: With more experience with SGLT2 inhibitors, we may rethink whether metformin should continue to be the first-line agent or whether we might start with SGLT2 inhibitors and add metformin. The weight loss and blood pressure reductions achieved with SGLT2 inhibitors are attractive adjunctive benefits in most patients with T2DM. The best order of treatments may not be the same for all patients.
Q: Do you think pioglitazone should be considered before a sulfonylurea even though this agent has been associated with an increased risk of fractures?
A: Sulfonylureas may have little to no role in the management of T2DM now that we have agents that do not adversely affect ß-cell function. We used sulfonylureas because for many years the options were limited, but these agents appear to accelerate insulin resistance. Pioglitazone is not a perfect drug [for example, because of the fracture risk], but its favorable effect on several cardiovascular risk factors makes it attractive in T2DM.
Q: Are you concerned that an Achilles’ heel may yet be found for the SGLT2 inhibitors?
A: Canagliflozin is now approved in the United States, so in addition to a large body of Phase III data, we are now collecting post-marketing data. This drug appears to be very clean. The SGLT2 inhibitors are not completely free of side effects, but they are well tolerated. The most common side effects, such as genital infections and symptoms related to intravascular volume, have been mild and readily treated.
Abstract 1103-P
Tofogliflozin Improves Insulin Resistance as Well as Glucose Tolerance by Ameliorating Fatty Liver and Obesity
Atsushi Obata, Naoto Kubota, Tetsuya Kubota, Hiroyuki Sato, Yoshitaka Sakurai, Masanori Fukazawa, Masayuki Suzuki, Kiyofumi Honda, Yoshiyuki Suzuki, Sachiya Ikeda, Kohjiro Ueki, Takashi Kadowaki
SGLT2 is mainly present in S1 segment of renal proximal tubule and accounts for approximately 90% of total urinary glucose reabsorption. To elucidate the anti-diabetic effects of SGLT2 inhibitors, we conducted long term administration of Tofogliflozin(Tofo), a novel SGLT2 inhibitor, to diet induced obese model mice. C57BL/6J mice were fed a high fat diet or a high fat diet which contains Tofo for 8 weeks. Although food intake was increased, body weight and fat mass were significantly decreased in Tofo treated mice. Blood glucose levels were significantly lower in Tofo treated mice with reduction of serum insulin levels. OGTT conducted after drug washout revealed improved glucose tolerance in Tofo treated mice with significantly lower insulin levels. HOMA-R was also significantly improved in Tofo treated mice. In addition, Tofo treated mice demonstrated elevated serum free fatty acid levels and diminished adipocyte size, which indicated accelerated lipolysis in Tofo treated mice. Reduced respiratory quotient and elevated serum ketone body suggested acceleration of ß-oxidation in Tofo treated mice.
In fact, CPT1a expression levels were significantly increased in the liver of these mice. Decreased gene expressions of SCD-1, FAS, DGAT-1 and DGAT-2 were consistent with reduced liver triglyceride contents. As food intake of Tofo treated mice was increased, we also conducted a pair feeding experiment by adjusting the total amount of daily food intake to exclude the influence of overdosing. The pair feeding experiment revealed more significant results compared to the ad libitum experiment.
Moreover, the expression levels of TNFa, MCP-1 and IL-6 in white adipose tissue were decreased in Tofo treated mice. In conclusion, Tofo may shift the energy consumption from glucose to lipid by increasing urinary glucose excretion and improve insulin resistance by reducing fat mass and liver triglyceride contents and ameliorating inflammation in white adipose tissue.
Commentary on abstract 1103-P
The importance of SGLT2 to blood glucose reabsorption in the kidney is well established, but the potential for this protein to mediate other effects is being explored in development programs with novel SGLT2 inhibitors. In this summary of experimental studies with tofogliflozin, SGLT2 inhibition was linked to down-regulation of pro-inflammatory cytokines in adipose tissue. In mice on a high fat diet, long-term tofogliflozin exposure appeared to shift energy consumption from glucose to lipids, reducing fat mass and liver triglyceride levels. An observed increase in serum free fatty acid levels was accompanied by a decline in adipocyte size. Although overall caloric intake was increased, body weight also declined. Perhaps most significantly, a reduction in insulin resistance suggested an overall improvement in glucose metabolism. The findings overall suggest SGLT2 may be part of a complex signaling system, which, when inhibited, has broader effects on glucose metabolism than blockade of reabsorption.
Questions and answers with Takashi Kadowaki, MD, PhD
Q: You described tofogliflozin as novel. How is it different than currently available agents?
A: Tofogliflozin has a high degree of selectivity for SGLT2 relative to SGLT1, SGLT6, and other sodium glucose co-transporter proteins. In studies we conducted, it produced the highest selectivity of the agents that are now in late stages of clinical development.
Q: The current explanation of the antidiabetic effect of SGLT2 inhibitors is that they block glucose reabsorption. Is this too simplistic?
A: There is evidence from other studies we have conducted with tofogliflozin that glucose physiology is complex, suggesting that SGLT2 may mediate signals of glucose metabolism other than glucose reabsorption.
Q: Your study also suggests effects on fat metabolism?
A: It is not clear whether these are direct or downstream effects. In mice on a high-fat diet, chronic tofogliflozin was associated with changes in liver gene expression that were consistent with the observed reduction in triglyceride production.
Q: Is it possible that features such as SGLT2 selectivity will differentiate SGLT2 inhibitors clinically?
A: Despite the fact that the development of SGLT2 inhibitors is sufficiently advanced that there are now agents approved for routine clinical use, I think we are still at an early stage of understanding the role played by these co-transporters in normal physiology as well as in metabolic diseases. The ability of these drugs to lower plasma glucose levels may not be the whole story in defining clinical effects.
Abstract 1104-P
Empagliflozin in Patients with Type 2 Diabetes Mellitus and Renal Impairment
Anthony H. Barnett, Ambrish Mithal, Jenny Manassie, Russell Jones, Henning Rattunde, Hans J. Woerle, Uli C. Broedl
A Phase III trial investigated the efficacy and safety of empagliflozin (EMPA) as add-on to existing therapy for 52 weeks in patients with T2DM and RI. Patients with mild RI (eGFR [MDRD equation] ≥60 to <90 mL/min/1.73 m2; n=290; mean age 62.6 years; mean BMI 31.5 kg/m2) received EMPA 10 or 25 mg qd or placebo (PBO). Patients with moderate RI (eGFR ≥30 to <60 mL/min/1.73 m2; n=374; mean age 64.9 years; mean BMI 30.2 kg/m2) received EMPA 25 mg qd or PBO. The primary endpoint was change from baseline in HbA1c at week 24. Exploratory endpoints included changes from baseline in fasting plasma glucose (FPG), weight and blood pressure (BP) at week 24. EMPA significantly reduced HbA1c vs PBO at week 24. Further analyses showed significant reductions in FPG, weight and BP. At week 24, adverse events (AEs) were reported by 79.6%, 75.4% and 72.7% of all patients (including an exploratory group with severe RI [n=74] on EMPA 25 mg or PBO) on EMPA 10 mg, 25 mg and PBO, respectively. Hypoglycemia (plasma glucose ≤70 mg/dL and/ or requiring assistance) was reported in 23.5% of patients on EMPA 10 mg, 22.1% on EMPA 25 mg and 22.9% on PBO. AEs consistent with urinary tract infection were reported in 10.2% of patients on EMPA 10 mg, 9.0% on EMPA 25 mg and 8.2% on PBO. AEs consistent with genital infection were reported in 6.1% of patients on EMPA 10 mg, 2.5% on EMPA 25 mg and 1.3% on PBO. To conclude, in patients with T2DM and mild or moderate RI, EMPA reduced HbA1c, weight, and BP vs PBO, and was well tolerated.
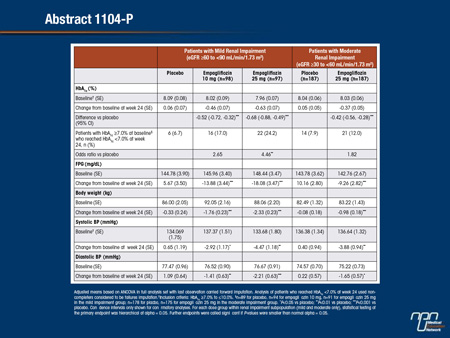
Commentary on abstract 1104-P
SGLT2 inhibitors reduce blood glucose by blocking a transporter of glucose reabsorption in the kidney. Impaired renal function predicts diminished activity of agents in this class, but the degree of renal impairment required to reduce the activity of SGLT2 inhibitors is not well established. In this study, enrollment was restricted to T2DM patients with eGFR <90 mL/min/1.73 m2. Patients with eGFR ≥60 mL/min/1.73 m2were randomized to 10 mg empagliflozin, 25 mg empagliflozin, or placebo. In patients with eGFR ≥30 but <60 mL/min/1.73 m2, the randomization was to 25 mg empagliflozin or placebo. The reduction in HbA1c at 24 weeks was 0.63% on the higher dose and 0.46% on the lower dose in those with mild renal impairment. In those with moderate impairment, the 25 mg dose of empagliflozin reduced HbA1c by 0.37%, which remained highly significant (P<0.001) relative to placebo. Volume depletion events were not more common on empagliflozin, but there were small decreases in eGFR at the end of 52 weeks of follow-up in both groups. When the inhibitor was discontinued, eGFR returned to baseline values within 3 weeks.
Questions and answers with Anthony H. Barnett, MD
Q: This study, like others, suggests that the blood-glucose lowering effects of SGLT2 inhibitors diminishes with diminishing renal function. Is there an eGFR below which SGLT2 inhibitors should not be used?
A: This appears to be an issue of efficacy, not safety. The HbA1c reductions relative to placebo remained significant in those with moderate renal impairment even though they were not as great as those achieved in patients with only mild impairment. In patients with moderate impairment, other antidiabetic therapies may be preferable. However, we did document antidiabetic activity even in a small exploratory group with eGFR <30 mL/min/1.73 m2included in our analysis.
Q: You reported diminished eGFR at 52 weeks in patients on empagliflozin. Does this suggest that these agents may contribute to progression of renal impairment in patients who have chronic kidney disease?
A: The reductions in eGFR were small and of uncertain clinical significance, but they do not appear to represent progressive renal insufficiency related to treatment. When the SGLT2 inhibitors were discontinued, the eGFR returned to baseline values within several weeks.
Q: Are the reductions in body weight and systolic blood pressure with SGLT2 inhibitors also dependent on preserved renal function?
A: The changes in body weight, which were reduced by about 1 kg in those with moderate renal impairment versus about 2.5 kg in those with mild renal impairment, appear to be more dependent than blood pressure reduction on preserved renal function. At 52 weeks, the reductions in systolic blood pressure were more similar, averaging about 5 mmHg in those with moderate renal impairment and 6 mmHg in those with mild renal impairment.
Q: Was renal function related to risk of adverse events?
A: The proportion of patients considered to have a drug-related adverse event was actually numerically lower in those with moderate than mild renal impairment, but the discontinuation rates for adverse events were similar across all groups, supporting the conclusion that concern about efficacy rather than safety should drive concern about offering SGLT2 inhibitors to individuals with renal impairment.
Abstract 1116-P
Response to Dapagliflozin by Baseline HbA1c in Head-to-Head Comparisons
Robert R. Henry, Alexander V. Murray, Michael A. Nauck, Katja Rohwedder, Eva Johnsson, Shamik J. Parikh, Agata Ptaszynska
Dapagliflozin (DAPA), a selective SGLT2 inhibitor, reduces hyperglycemia by removing excess blood glucose via the urine. The objective of this analysis was to directly compare the glycemic efficacy of DAPA with other oral antidiabetic drugs (OADs) across a wide range of baseline HbA1c. DAPA was compared with other OADs in 2 randomized, double-blind clinical trials: Study 1 (NCT00660907), a 52-week trial of DAPA (≤10 mg) vs glipizide (GLIP, ≤20 mg) as add-on to metformin (MET); Study 2 (NCT00859898), a 24-week trial of DAPA 10 mg vs MET extended release 2000 mg as monotherapies. Mean baseline HbA1c was 7.7% (Study 1) and 9.1% (Study 2). Non-inferiority of DAPA to comparators for changes in HbA1c (primary endpoint) was previously described. Here we present analyses by baseline HbA1c levels (Figure). Both DAPA and comparators provided greater reductions in HbA1c in the higher baseline HbA1c subgroups.
Furthermore, within each study, reductions by DAPA and comparator were similar for each baseline HbA1c category. In both studies, DAPA had a low intrinsic propensity for hypoglycemia (Study 1: DAPA 3.5% vs GLIP 40.8%; Study 2: DAPA 0.9% vs MET 2.9%), consistent with the insulin-independent mechanism of action. In summary, as with the overall populations, DAPA demonstrated glycemic responses similar to other OADs in subgroups stratified by baseline glycemic control, producing larger reductions in HbA1c for individuals starting with higher baseline levels.
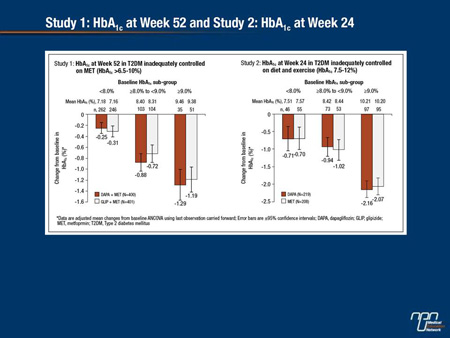
Commentary on abstract 1116-P
The goal of this study was to compare reductions in HbA1c on dapagliflozin relative to other oral antidiabetic agents at different starting HbA1c levels. The analysis included 2 studies. In one, dapagliflozin was compared to glipizide when both were added to metformin. In the second, dapagliflozin and an extended-release form of metformin were compared as monotherapies. In both studies, the reductions in HbA1c in the two arms were comparable overall with greater relative reductions achieved with each agent as the baseline HbA1c increased. The differences in the rates of hypoglycemia were striking, particularly in the study comparing the SGLT2 inhibitor to the sulfonylurea (3.5% vs. 40.8%, respectively). While the rate of hypoglycemia on metformin monotherapy was only 2.9%, the rate was 0.9% on dapagliflozin. As expected, genital infection rates were higher on dapagliflozin in both the combination study (10.8% vs. 1.7%) and the monotherapy study (9.6% vs. 0.5%). One or more serious adverse events was reported in 8.6% of those on dapagliflozin plus metformin, 11.3% of those on glipizide plus metformin, 2.3% of those on dapagliflozin monotherapy and 1.9% of those on metformin monotherapy.
Questions and answers with Robert Henry, MD
Q: Has the greater relative reductions in HbA1c in those with higher versus lower baseline levels been a consistent finding in SGLT2 inhibitor studies overall?
A: Yes, this has been reported previously in placebo-controlled trials, but the studies reported here looked at the relative reductions at different levels of HbA1c when dapagliflozin was compared to another strategy. The data suggest that all of the tested agents provide a greater relative reduction in HbA1c as HbA1c increases.
Q: Why was hypoglycemia a major point of comparison?
A: Obviously, effective glucose lowering is important in the treatment of diabetes, but hypoglycemia is the flip side of efficacy. While the SGLT2 inhibitor and the sulfonylurea provided similar reductions in HbA1c even in those with high baseline levels, dapagliflozin did so with a low risk of producing hypoglycemia.
Q: On dapagliflozin therapy, there was about a 10% rate of genital infections and an 8% rate of urinary tract infections. This is almost twice as high as those reported for most other SGLT2 inhibitors at this meeting. Is there any reason?
A: There is no reason to suspect that dapagliflozin is associated with a higher risk of these infections. There are a lot of possible explanations, including differences in the study populations. I do not think this result is remarkable.
Q: What are the key messages from this study?
A: The magnitude of efficacy of dapagliflozin, like other commonly used oral antidiabetic agents, is dependent on the baseline HbA1c levels whether it is used alone or in combination. All of these drugs were well tolerated, but dapagliflozin and metformin share a low risk of causing hypoglycemia.
Abstract 1139-P
Urinary Tract Infection (UTI) With Canagliflozin (CANA) in Subjects with Type 2 Diabetes Mellitus (T2DM)
Lindsay E. Nicolle, George Capuano, Albert Fung, Keith Usiskin
CANA, an SGLT2 inhibitor, inhibits renal glucose reabsorption, resulting in increased glucosuria. The impact of this on UTI was assessed in subjects with T2DM in placebo (PBO)-controlled, Phase 3 studies of CANA using a UTI case report form to characterize the presentation and severity of events. Findings are reported for pooled analyses of subjects from four 26-wk studies (DS1; N = 2,313) and subjects with moderate renal impairment (eGFR ≥30 and <60 mL/min/1.73 m2) from four 18- or 26-wk studies (DS2; N = 1,085), and for the CANagliflozin cardioVascular Assessment Study (CANVAS; 52-wk interim safety analysis) in subjects with a history or high risk of cardiovascular disease (N = 4,327); subjects with a history of UTI were not excluded from these studies. Across datasets analyzed, the proportion of subjects with UTI was slightly higher with CANA than PBO, with no increase in recurrent or serious events or in UTI-related discontinuations; upper UTI rates were low and similar across groups. UTI was more common in women than men, but the increased incidence with CANA was consistent between genders. UTIs generally occurred within 26 wks of starting CANA, with a subsequent attenuation in incidences. Median time to first symptomatic UTI tended to be earlier with CANA, but median duration and severity of symptoms of UTIs were similar with CANA and PBO. In summary, a slight increase in UTI rates, with no increase in serious or upper UTI, was seen with CANA in subjects with T2DM.
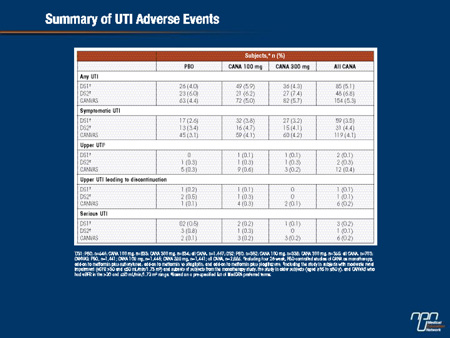
Commentary on abstract 1139-P
SGLT2 inhibitors eliminate glucose in the urine, increasing urinary sugar content. The high rate of genital infections associated with SGLT2 inhibitors in Phase III trials is attributed to mycotic growth secondary to this effect. However, there is also a potential for glycosuria to induce urinary tract infections (UTIs) caused by bacteria or other pathogens. In this study, the goal was to characterize the incidence of UTIs from a data pool of more than 5,000 patients participating in trials of canagliflozin. Overall, symptomatic UTIs were observed in approximately 4% of patients treated with canagliflozin and approximately 3% of those treated with placebo. The rates of serious UTIs or UTIs leading to therapy discontinuation were not significantly different in these two groups. The rates of upper UTIs were low and similar in the active treatment and placebo groups. While the rate of UTIs was greater overall in women than men, the small relative increase on canagliflozin was consistent across genders. It is notable that the increased rate of UTIs was observed primarily within the first 26 weeks of treatment.
Questions and answers with Keith Usiskin, MD
Q: The study suggests a 1% absolute difference in the rate of UTIs. Is this clinically meaningful?
A: The conclusion from this large dataset is that there is a slightly higher rate of UTI on canagliflozin. Is a 1% difference clinically meaningful? I am not sure there is an objective answer.
Q: Do you believe the mechanism of UTIs is different from that of genital mycotic infections?
A: They are probably related. Urine with increased sugar content is likely to change the microenvironment that favours the growth of pathogens.
Q: Do you think that the diminishing risk of UTI over time is related to patients simply improving hygiene?
A: The UTIs appear to be readily treated. The question not yet answered is whether any prophylactic steps, such as more frequent washing, might reduce risk.
Q: Increased rate of genital infections has been seen consistently in the Phase III trials with SGLT2 inhibitors. Do you think that the small increased rate of UTIs observed in this study is also a class effect?
A: This is the largest dataset to evaluate UTIs on SGLT2 inhibitors of which I am aware. I think that the same results would be generated with any drug in this class. These are not serious events, but they can be bothersome.