Reports
New Insights into Strategies for Management of Crohn’s Disease and Ulcerative Colitis
This report is based on medical evidence presented at sanctioned medical congress, from peer reviewed literature or opinion provided by a qualified healthcare practitioner. The consumption of the information contained within this report is intended for qualified Canadian healthcare practitioners only.
MEDICAL FRONTIERS - 9th Congress of the European Crohn's and colitis Organisation (ECCO-IBD 2014)
Copenhagen, Denmark / February 20-22, 2014
Copenhagen - Numerous clinical trials have demonstrated the therapeutic value of treatment with monoclonal anti-TNF-a antibodies such as infliximab and adalimumab in luminal and/or fistulizing Crohn’s disease or ulcerative colitis, both as induction and as maintenance treatment. However, questions have remained about whether the benefits seen under clinical trial conditions could also be achieved in patients treated in community gastroenterology practices. The results of an international, community-based trial presented at ECCO 2014 showed that results with an accelerated treatment approach applied in academic centers can be reproduced in real-world practice. New data presented from extension trials of TNF-a blockers also reconfirmed the long-term efficacy and safety of these agents, while it was also shown that earlier identification of patients at increased risk of relapse while on maintenance treatment is possible.
Chief Medical Editor: Dr.Léna Coïc, Montréal, Quebec
Conventional treatment strategies in Crohn’s disease (CD) with corticosteroids, antimetabolites, and TNF-a blockers are focused on sequential and incremental treatment intensification based on symptoms, but they are frequently ineffective in moderate-to-severe disease. An approach involving the introduction of combined immunosuppression earlier in high-risk patients (accelerated step-care), has been shown to induce and maintain remission, reduce the use of corticosteroids, and heal intestinal ulceration in patients who fail conventional treatment. However, adoption of the accelerated step-care approach by community gastroenterologists has been relatively slow, primarily due to concerns that the safety and efficacy of these agents seen in clinical trials might not be generalizable to their practices.
Accelerated Treatment in CD
One of the highlights of the Congress was the presentation of the final results of the Randomized Evaluation of an Algorithm for Crohn’s Treatment (REACT) trial, showing that introduction of combined therapy earlier in real-world practice is feasible and safe and more effective than conventional management for the prevention of CD-related complications, surgeries, and hospitalizations (Abstract OP004). The community-based, cluster randomized trial, led by Dr. Brian Feagan, CEO and Director of Clinical Trials at Robarts Research Institute, Western University, London, Ontario, involved 34 gastroenterology practices in Canada and 6 in Belgium. “One of the main reasons for doing it was to see whether we could get around the ‘fear factor’ of using these drugs in community practice,” Dr. Feagan said. The practices were randomly assigned to either implement an accelerated step-care algorithm that featured use of combined adalimumab/antimetabolite therapy (intervention group) (Figure 1) earlier or to continue with their usual care for the management of CD (conventional management). Patients could enter at any point within the accelerated treatment algorithm. Patients with active disease were treated with corticosteroids and, unlike usual step-care, if they were not in symptomatic remission after 4 weeks, they were given combined TNF-a blocker (usually adalimumab) plus azathioprine plus methotrexate, according to physician judgment. Up to 60 consecutive adult patients were entered at each practice and evaluated for 24 months at each practice. In total, approximately 900 patients were managed conventionally and over 1000 received early combined immunosuppression.
Figure 1.
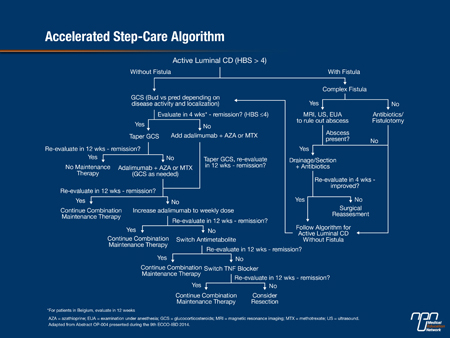
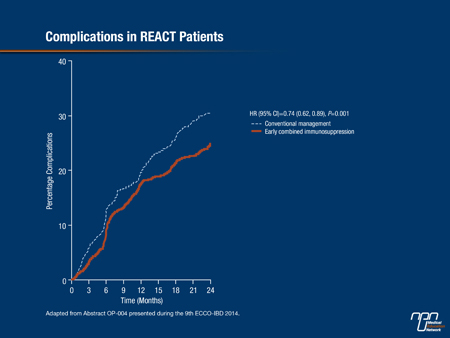
The results for the primary outcome of the trial—the proportion of patients in corticosteroid-free remission (Harvey Bradshaw Score ≤4) at 12 months—showed a modest increase in the group assigned to accelerated step-care (66%) vs. conventional management (62%). Dr. Feagan noted, “the rate was consistently higher in the intervention group over the entire period of follow-up,” he stressed. However, “there was quite a different story when we looked at the secondary endpoints.” Statistically significant and clinically important reductions with accelerated treatment were seen in time to first surgery (32%) (Figure 2) and time to first complications (26%) (Figure 3), and there was also a 16% reduction in time to first hospitalization. “A combined aggregate endpoint of ... hospitalization, surgery, or complication showed a clear separation between the 2 groups,” Dr. Feagan reported, with a significant 26% reduction associated with accelerated treatment (P<0.001). These results were consistent with those of the CHARM trial, which showed reduced 1-year risks of hospitalization and surgery with adalimumab vs. placebo in a much higher risk population, Dr. Feagan noted (Feagan BG et al. Gastroenterology 2008;135:1493-9).
The important question of safety did not differ between the two treatment groups, with low rates of serious infections, Dr. Feagan reported. Almost all deaths that occurred were in elderly people “This is an important message,” he stated. “There weren’t deaths in young people; there weren’t large numbers of opportunistic infections. This is a very positive story.” One of the constraints on the use of the accelerated therapeutic approach in the community had been fear of infection, he noted. “There is also an inference from these data that symptoms may not be the best way of monitoring patients and guiding therapy in practice,” he added. “I think we have learned that treating symptoms for this disease is a waste of time. We have to have objective measures,” he declared.
A follow-up trial, REACT 2, based on the same design as the first REACT trial and also led by Dr. Feagan, will involve a smaller number of practices and patients: 15 practices, with 40 patients per practice. REACT 2 will use endoscopy to evaluate accelerated treatment. “We think endoscopy will provide much more differentiation and a bigger effect size,” Dr. Feagan commented.
Figure 2.
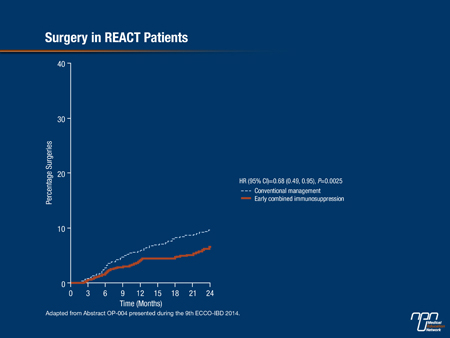
Figure 3.
Pediatric CD
A new analysis of IMAgINE 1 trial data presented by Dr. Anne Griffiths from The Hospital for Sick Children, Toronto, showed that one third of adalimumab-treated children taking corticosteroids at the start of the trial achieved corticosteroid-free remission at 26 and 52 weeks (Abstract OP021). The main IMAgINE trial was the basis for approval for pediatric use in CD. Patients with CD diagnosed for >12 weeks and Pediatric Crohn's Disease Activity Index (PCDAI) >30 received open-label induction therapy with adalimumab at weeks 0 and 2 by body weight and randomized at week 4 to double-blind higher-dose adalimumab (<40 kg, 20 mg every other week (eow); ≥40 kg, 40 mg eow) or lower-dose adalimumab (<40 kg, 10 mg eow; ≥40 kg, 20 mg eow) for 48 weeks. Among the 71 patients randomized to adalimumab who were using corticosteroids at baseline, corticosteroid-free remission (PCDAI ≤10) was achieved in 26% of the low-dose and 33% of the high dose adalimumab groups at 26 weeks, and 18% and 27%, respectively, at 52 weeks. Baseline disease severity did not appear to affect corticosteroid-free remission rates. “The patients in whom adalimumab was the first anti-TNF drug did significantly better,” Dr. Griffiths noted, with 52% and 41% of infliximab-naïve patients on high-dose adalimumab in remission at weeks 26 and 52, respectively. Time to remission in these patients was less than 3 months. Dr. Griffiths suggested that the effect of adalimumab may have been greater than observed. “I suspect that there are issues with the PCDAI that led to the results appearing as modest as they do, particularly in the infliximab experienced group,” she commented.
Long-term Safety in CD
Follow-up of patients in the IMAgINE 1 trial has now continued for over 5 years, and the latest analysis, presented by Dr. Joel Rosh (Goryeb Children’s Hospital/Atlantic Health, Morristown, New Jersey, USA), showed that adalimumab continues to be well-tolerated in children with moderately-to-severely active CD, with no new safety risks identified (Abstract P426). As of June 30, 2013, data for 192 patients, representing 422.2 patient years of exposure were available, with a mean duration of exposure of 2.2 years. Adverse events (AEs) and serious adverse events (SAEs) leading to discontinuation were mostly worsening of CD. Most opportunistic infections (7 events) were non-serious oral candidiasis, and hematologic AEs were mostly anemia (22 events). No deaths, demyelinating disease, malignancies, or tuberculosis have been reported. The rate of SAEs and AEs, and AEs leading to discontinuation were significantly higher for infliximab-experienced than infliximab-naïve patients (P<0.001). Serious infections were reported by 22 (11.5%) patients. Overall, the exposure-adjusted rate AEs was similar between patients receiving adalimumab with or without concomitant immunomodulator, but those using concomitant corticosteroids experienced more SAEs than patients without concomitant corticosteroids, Dr. Josh noted.
Predicting Relapse in CD
“Highly accurate prediction of relapse in CD patients on treatment with adalimumab can be achieved by measuring fecal calprotectin,” reported Dr. Rocio Ferreiro, University Hospital Santiago de Compostela, Santiago, Spain. Using a rapid test (Quantum Blue®, Bühlmann), Dr. Ferreiro and her colleagues measured fecal calprotectin on the day of the first adalimumab injection in 30 CD patients who had been in clinical remission for ≥6 months on treatment with a continuous standard dose of adalimumab 40 mg eow (Abstract P107). After 4 months of follow-up, 70% of patients remained in clinical remission while 30% had had a relapse. Fecal calprotectin concentration at inclusion was significantly higher in the patients who relapsed than in those who remained in remission (mean 803 vs. 95 μg/g; P<0.005). The ability to predict relapse with this non-invasive biomarker could allow for early changes in treatment in these patients, Dr. Ferreiro suggested.
Moderate-to-severe UC
Higher rates of subsequent UC-related hospitalizations occur in adalimumab-treated patients with active UC and Mayo subscore 2 or 3 compared with patients with Mayo subscore values of 0 or 1, according to a study presented by Dr. Walter Reinisch (Department of Medicine, McMaster University) (Abstract P552). The study involved over 400 patients who were enrolled in the ULTRA 1 or ULTRA 2 trials and had Mayo subscores at Week 8. In these two large randomized trials, adalimumab (160/80 mg at weeks 0/2 followed by 40 mg eow) was shown to be effective in inducing and maintaining remission up to 52 weeks in patients with moderately to severely active UC (Reinisch W et al. Gut 2011; 60:780-7; Sandborn WJ, et al. Gastroenterology 2012;142:257-65). Adalimumab therapy was associated with significant reductions in the risks of all-cause, UC-related, and UC- or drug-related hospitalizations compared with placebo (Feagan BG et al. Gastroenterology 2014;146:110-8). In the latest analysis of ULTRA 1 and 2 data, the correlation with UC-related hospitalizations was significant for Mayo subscore values of rectal bleeding, physician’s global assessment, and endoscopy. Similar findings were observed for the incidence rate for total events of UC-related hospitalizations and for all-cause hospitalizations.
More than half (58%) the patients from the ULTRA 1 and ULTRA 2 trials are being followed in the open-label extension study, ULTRA 3, in which patients received adalimumab 40 mg eow or weekly. The latest update from ULTRA 3, reported by Dr. Jean-Frédéric Colombel, Icahn School of Medicine, Mount Sinai Medical Center, New York, showed that remission and mucosal healing rates have been maintained at 51% and 46%, respectively, for up to 4 years, with a stable safety profile and no new safety signals (Abstract P571).
Summary
The data presented at ECCO support the use of accelerated step-care as a best practice in the treatment of active CD and should provide reassurance for community gastroenterologists. Extension trials of TNF-a blocker treatment in CD and UC continue to confirm the long-term efficacy and safety of treatment with adalimumab.
MF1