Reports
New Oral Oncolytics: Major Step Forward in the Management of Metastatic Castrate-Resistant Prostate Cancer
This report is based on medical evidence presented at sanctioned medical congress, from peer reviewed literature or opinion provided by a qualified healthcare practitioner. The consumption of the information contained within this report is intended for qualified Canadian healthcare practitioners only.
PHYSICIAN PERSPECTIVE - Viewpoint based on presentations from the 108th Annual Meeting of the American Urological Association
San Diego, California / May 4-8, 2013
Reviewed and edited by:
Fred Saad, MD, FRCS
Professor and Chief of Urology
Director of Urologic Oncology
U of M Endowed Chair in Prostate Cancer
University of Montreal Hospital Centre
Université de Montréal
Montreal, Québec
Introduction
One of the newer concepts that governs current management of mCRPC is the idea that androgens are produced not only by the testes but by the adrenals and, more importantly, by the tumour itself. Even at the castrate level, extragonadal androgens circulate in quantities that are sufficient enough to stimulate androgen receptors that remain active in most patients with CRPC. Once prostate cancer (PC) progresses despite androgen deprivation therapy, it becomes resistant to castration levels of androgen and hormonal therapy is no longer effective. Prostate-specific antigen (PSA) levels invariably rise and with time, metastases begin to appear. Agents that completely inhibit androgen signalling or production even at the extragonadal level are now available and are a major step forward in the management of mCRPC.
By blocking the critical CYP17 enzyme in testosterone synthesis, abiraterone acetate blocks all sources of androgen production including androgen biosynthesis by the adrenal glands, the testes and by the tumour itself. As PC tumour growth is also dependent on androgen receptor (AR) signalling, enzalutamide effectively shuts down prostate cancer proliferation and dissemination by blocking AR signalling.
Inhibition of Androgen Biosynthesis
In the first study of androgen biosynthesis inhibition by abiraterone acetate (COU-AA-301), de Bono et al. (N Engl J Med 2011;364:1995-2005) reported that 1000 mg/day plus prednisone 5 mg twice a day prolonged overall survival (OS) among men with mCRPC who had previously received chemotherapy with docetaxel. In order to attenuate mineralocorticoid-like effects that arise from an increase in aldosterone production when abiraterone is given on its own, prednisone is always co-administered with it.
A second study (COU-AA-302) by Ryan et al. (N Engl J Med 2013;368:138-48) used the same dosing schedule in chemotherapy-naive mCRPC patients. The COU-AA-302 study was unblinded after 43% of the expected deaths had occurred. Median radiographic progression-free survival (rPFS) was 16.5 months with abiraterone/prednisone vs. 8.3 months with placebo/prednisone (HR 0.53; 95% CI, 0.4-0.62; P<0.001). Over a median follow-up of 22.2 months, median OS was not reached with abiraterone/prednisone vs. prednisone alone (27.2 months) but it did not reach the prespecified P-value for statistical significance (HR 0.75; 95% CI, 0.61-0.93; P=0.01).
COU-AA-302 Third Interim Analysis
At the AUA, the COU-AA-302 third interim analysis—when 55% of deaths had occurred—was presented by Dr. Fred Saad from the University of Montreal. The study involved approximately 1100 asymptomatic or mildly symptomatic mCRPC patients at baseline. The co-primary end points were rPFS and OS.
Patient characteristics included a median baseline PSA level of approximately 40 ng/mL; 80% had bone metastases; and approximately half had over 10 bone metastases on study entry. Nearly half also had soft tissue measurable lesions. The study duration was 27.1 months and those randomized to the abiraterone/prednisone arm received a median of 15 cycles. Those on prednisone alone received a median of 9 cycles.
Final analysis showed that treatment with abiraterone virtually doubled the time to rPFS (16.5 months vs. 8.3 months for prednisone alone, a 47% reduction in the risk of disease progression over steroids alone (P<0.0001). Moreover, OS improved with abiraterone at 35.3 months vs. 30.1 months for prednisone alone, a 21% reduction in the risk of death with this novel agent (P of 0.0151 did not reach the prespecified value of 0.0035 for statistical significance) (Figure 1).
Figure 1. COU-AA-302 OS Results
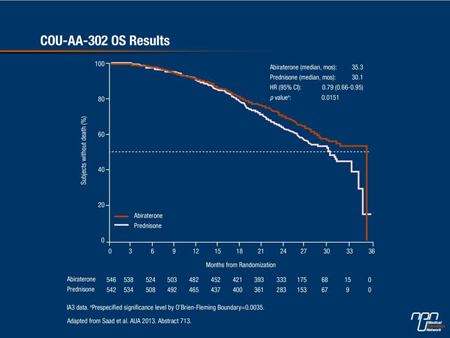
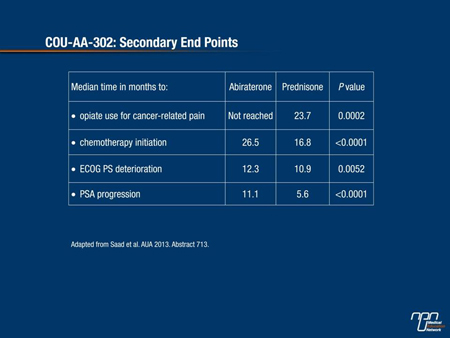
Of major importance to patients in terms of quality of life until disease progression, secondary end points were all significantly prolonged with active treatment vs. prednisone alone (time to opiate use, time to initiation of chemotherapy and time to ECOG performance status deterioration (Table 1). Notably, in the abiraterone/prednisone arm, 69% of patients had a decline in PSA level ≥50% compared to 29% of those on prednisone alone.
Table 1. COU-AA-302: Secondary End Points
Even with almost double the exposure time to abiraterone, the safety and tolerability profile remained favourable and similar to those observed in the COU-AA-301 study. As a result of these findings, the 2013 AUA guidelines presented at the meeting now recommend that abiraterone + prednisone, docetaxel or sipuleucel-T (not available in Canada) be offered to patients with asymptomatic or minimally symptomatic mCRPC with good performance status and no prior docetaxel chemotherapy.
Pain and Functional Status
A separate aspect of the study reported by Dr. Neal Shore, Director, Carolina Urologic Research Center, Myrtle Beach, South Carolina, focused on pain and functional status in the two treatment arms of COU-AA-302. A statistically significant improvement in pain interference (P=0.005) was documented in the abiraterone/prednisone arm vs. prednisone alone.
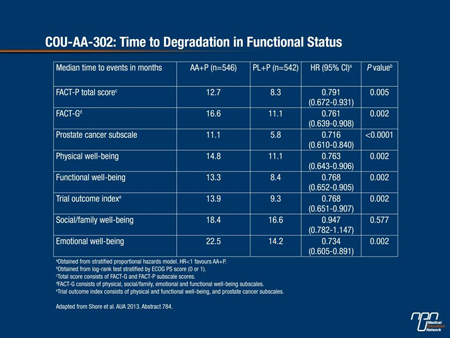
A post-hoc analysis presented here at the AUA also demonstrated that abiraterone/prednisone significantly delayed worst pain progression (P=0.035) when evaluated using a 2-point change in item 3 of the Brief Pain Inventory-SF (BPI-SF) scale. The authors suggested that a 2-point change threshold in the worst pain on this scale may be more clinically relevant to assess a study population where a high proportion of patients had no or minimal pain at baseline. The same analysis also showed that the active treatment strategy significantly delayed degradation in the Functional Assessment of Cancer Therapy—Prostate (FACT-P) scale (P=0.005) and in the prostate cancer-specific subscale vs. prednisone alone (P<0.0001) (Table 2).
Table 2. COU-AA-302: Time to Degradation in Functional Status
At the time of final analysis, the addition of bone-targeted therapy showed no trend towards improvement in the primary end points. Nevertheless, improvement in symptomatic progression in each of the secondary end points was observed with the use of bone-targeted therapy. Grade 3/4 adverse events (AEs) were slightly more common in the active treatment arm but they were always reversible and usually occurred within the first 3 months of treatment.
The final results of the COU-AA-302 trial thus demonstrate that the oral androgen biosynthesis inhibitor abiraterone acetate is both safe and well tolerated in men with mCRPC over a significant treatment interval.
Assessing Tissue and Serum Effects of Combination Androgen Deprivation Therapy
In an innovative study, Richie et al. assessed tissue and serum effects of abiraterone in combination with androgen deprivation therapy for localized, high-risk prostate cancer prior to prostatectomy (Abstract 665).
All patients received 24 weeks of androgen deprivation therapy and were randomized to abiraterone 1000 mg/day, prednisone 5 mg/day and a luteinizing hormone-releasing hormone analogues (LHRHa) (either 22.5 mg IM q12 weeks or 7.5 mg IM q4 weeks) or to the LHRHa alone for the first 12 weeks.
At the completion of 12 weeks of treatment, all patients underwent ultrasound-guided prostate biopsies to measure tissue hormone. All patients received an additional 12 weeks of LHRHa/abiraterone followed by radical prostatectomy at 24 weeks. Thus, one group received abiraterone during the final 12 weeks of the study (Group 12/24) whereas the other group received it for the 24 weeks (Group 24/24).
As reported by investigators here, median PSA was markedly lower in the 24/24 group at a median of 0.10 ng/Ml at 12 weeks compared with a median of 1.06 ng/mL in the 12/24 group. In fact, only 1 patient out of 28 achieved a PSA ≤0.2 ng/mL at week 12 in the group who received the LHRHa alone compared with 26 out of 29 patients who received abiraterone from randomization onwards.
Intraprostatic tissue and serum androgen results also showed that the combination of LHRHa/abiraterone/prednisone significantly lowered both intraprostatic and serum androgens compared with LHRHa treatment alone. The LHRHa/abiraterone/prednisone regimen was also well tolerated with low rates of systemic toxicity. Complication rates during subsequent radical prostatectomy were not higher in the group who received prior treatment with abiraterone plus prednisone, as investigators also pointed out.
Researchers concluded that extragonadal androgen suppression in early treatment paradigms may lead to improved clinical outcomes in patients with localized high-risk PC.
Blocking AR signalling: AFFIRM Results
Blocking AR signalling with enzalutamide has also produced robust clinical responses in mCRPC. According to phase III AFFIRM trial results (N Engl J Med 2012;367:1187-97), enzalutamide increased median OS by 4.8 months (P<0.001) and median rPFS by 5.4 months (P<0.001) compared with placebo in men with mCRPC who had previously received docetaxel. It also increased time to PSA progression by 5.3 months (P<0.001). A post-hoc analysis of the impact of baseline PSA levels on outcomes in AFFIRM was presented by Saad et al.
Patients were separated into baseline PSA level quartiles: <40.2 ng/mL; 40.2 to <111.2 ng/mL; 111.2 to <406.2 ng/mL and ≥406.2 ng/mL. Regardless of how advanced disease was at the time of study entry, enzalutamide provided a consistent relative benefit in time to event outcomes with a reduction in mortality ranging between 45% and 47% across all 4 PSA quartiles (Table 3).
Table 3. AFFIRM: OS Results in Months by Baseline PSA Level
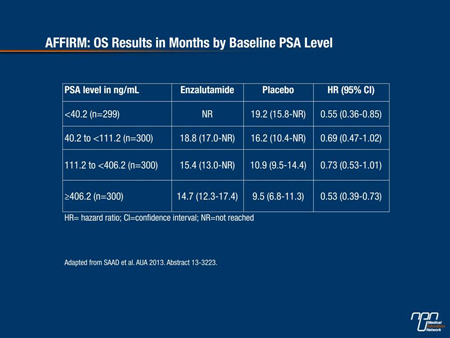
Men who entered the study with a PSA ≥400 ng/mL had much shorter survival times, at about half those who entered the study with a PSA <40 ng/mL.
Results confirm that enzalutamide is a viable treatment option for mCRPC patients following docetaxel chemotherapy regardless of PSA levels.
Treatment Options in Non-metastatic Disease
Important strides have clearly been made in the setting of metastatic PC. However, if patients have castrate-resistant disease with no evidence of metastases, no treatment is yet indicated until PSA levels begin to rise. How quickly PSA levels rise dictate the frequency of follow-up as the faster the PSA level rises, the faster metastatic disease develops.
New Canadian guidelines indicate that men with CRPC but no metastases should undergo diagnostic imaging every 3 to 6 months if they are experiencing a rapid rise in PSA and once a year if their PSA is rising more slowly.
Another group of patients for whom treatment options are not usually curative are young patients who have very aggressive localized disease. For these patients, a compilation of studies from the 1990s and the early 2000s failed to show any improvement in the rates of PSA relapse with androgen deprivation therapy using either LHRHa alone or in combination with antiandrogens.
Other Novel Approaches
Another treatment option is sipuleucel-T, a “tailor-made” vaccine obtained by leukapheresis of a patient’s blood. The blood is then cultured with a recombinant human protein consisting of prostatic acid phosphatase linked to granulocyte-macrophage colony-stimulating factor. Sipuleucel-T was the first immunotherapeutic agent to be approved in the US based on observed improvements in OS compared with placebo.
On the other hand, a confirmatory trial involving 512 men with mCRPC found no difference in PSA response or PFS in favour of the vaccine (N Engl J Med 2010;363:411-22). The vaccine is also expensive and complicated to produce and is thus unlikely to become another treatment option in mCRPC at least here in Canada any time soon.
Another novel approach actively being pursued in the setting of mCRPC is an antisense oligonucleotide targeting clusterin using custirsen (OGX-011). As reported by Chi et al. (J Clin Oncol 2012;28:4247-54), custirsen given weekly with docetaxel/prednisone in chemotherapy-naive patients resulted in a median OS of 23.8 months vs. 16.9 months to those treated with docetaxel/prednisone alone in a phase II of mCRPC. By inhibiting clusterin, it is hoped that cancer cells can be made more sensitive to docetaxel.
Results from a just completed phase III international trial evaluating this novel new oligonucleotide are eagerly awaited.
Summary
PC is the third most common cause of cancer-related death among Canadian men with some 4000 deaths each year. Currently, most treatment options for mCRPC are indicated only in the post-chemotherapy setting following treatment with docetaxel. Because of toxicity concerns, chemotherapy is not an option for many patients with mCRPC. With several newly and likely to be approved oral oncolytics for mCRPC, patients with mCRPC may receive treatment before docetaxel chemotherapy as well as after. Abiraterone and enzalutamide offer new pathways by which to achieve more profound androgen suppression than has ever been possible before and with it, real gains in survival and quality of life for men with mCRPC. The availability of these new oral oncolytics will also allow urologists who are interested in the management of mCRPC patients to become more directly involved in their care.