Reports
New Standard for Hepatitis C Treatment
This report is based on medical evidence presented at sanctioned medical congress, from peer reviewed literature or opinion provided by a qualified healthcare practitioner. The consumption of the information contained within this report is intended for qualified Canadian healthcare practitioners only.
MEDICAL FRONTIERS - Canadian Digestive Diseases Week
Montréal, Quebec / February 24-27, 2012
Montréal - The burden of hepatitis C viral (HCV) disease is growing. At present, about 250,000 individuals in Canada are infected. An estimated 8000 to 10,000 new cases emerge each year, the majority of which are genotype 1. Many cases of HCV remain undiagnosed until potentially life-threatening complications arise. Treatment that achieves a sustained virologic response (SVR) in a large proportion of patients has remained elusive. Triple therapy, which involves adding a protease inhibitor to pegylated interferon and ribavirin, has substantially increased SVR rates. New guidelines for HCV treatment recommend greater attention to screening for HCV and consideration of triple therapy for infected patients.
Chief Medical Editor: Dr. Léna Coïc, Montréal, Quebec
Current epidemiologic trends, including a rising rate of infection in young adults, suggest that there will be a progressive increase in hepatitis C viral (HCV) disease and sequelae including cirrhosis and carcinoma. “The number of patients presenting with end-stage liver disease is increasing, and will probably increase for the next 10 to 20 years,” remarked Dr. Robert Myers, University of Calgary, Alberta.
These findings emphasize the importance of reducing infection through improved diagnosis and uptake of effective therapy. “We haven’t diagnosed enough and we are not treating enough patients; until recently, [treatment response] rates have not been high enough…We [now] have more effective therapies but we need to increase the number of patients that we are treating….Our approach has been to screen people with risk factors or elevated ALT and clearly that’s not getting us anywhere. We need to take a more population screening-type approach,”
Dr. Myers told delegates.
Updated Canadian Guidelines
New guidelines for the management of HCV, now being finalized by the Canadian Association for the Study of the Liver (CASL), will recommend development of strategies for case identification, Dr. Myers noted. They will also emphasize the need to expand the capacity for and expertise in HCV diagnosis and management among Canadian health care providers.
The CASL guidelines recommend that all patients with HCV, especially those with fibrosis, be considered for antiviral therapy. However, several variables may influence the initiation or timing of therapy. “Factors to consider include the probability of SVR in a given patient, the likelihood of progression to advanced disease within their lifetime—so considering comorbidities and life expectancy, [the] anticipated tolerability of treatment, [and] extrahepatic manifestations which would suggest earlier treatment. Of course, many patients have concerns regarding infectivity, which may prompt early treatment [even if the disease is mild],” Dr. Myers indicated.
Improving Standard Therapy
The goal of HCV treatment is eradication of the virus, defined by undetectable serum HCV RNA on an assay with a lower limit of detection of 10 IU/mL, 24 weeks after treatment (sustained virologic response [SVR]). An SVR is associated with a lower rate of clinical events, including mortality, and resolution of hepatic fibrosis, indicated Dr. Adrian Di Bisceglie, Saint Louis University School of Medicine, Missouri.
For about the last 10 years, standard therapy for genotype 1 HCV has been the combination of pegylated interferon and ribavirin (PR), which leads to SVR in about 40% of treated patients. Dr. Di Bisceglie stated that there has been “real excitement” about the introduction of direct-acting antiviral agents, including the NS3/4A protease inhibitors (PIs) telaprevir and boceprevir. When added to PR, these oral medications substantially increase SVR rates in both treatment-naive and treatment-experienced patients. “It is indeed a new era in the management of hepatitis C,” he remarked. As several speakers emphasized, the new PIs cannot be used as monotherapy in HCV as they may select for resistance.
ADVANCE in Triple Therapy
Several phase III studies have confirmed the efficacy of triple therapy for genotype 1 HCV. This involves the addition of a PI during part of the PR treatment regimen. Started concurrently with PR, telaprevir is given for 12 weeks and is followed by a further 12 or 36 weeks of PR depending on patient profile (i.e. absence or presence of cirrhosis) and/or HCV RNA response to therapy. Boceprevir is given for 24, 32 or 44 weeks after a 4-week initial period of PR is required, then concomitantly with PR. A further 12 or 20 weeks of PR may be required according to patient profile or response.
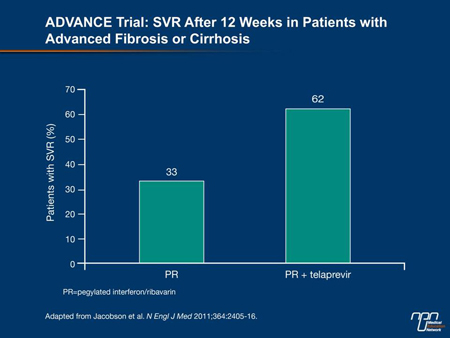
The ADVANCE trial (Jacobson et al. N Engl J Med 2011;364:2405-16) compared combination telaprevir/PR with PR alone in 1088 treatment-naive patients. The administration of telaprevir for 8 or 12 weeks produced significantly higher SVR rates (69% and 75%, respectively) than PR alone (44%). Of interest, commented Dr. Paul Marotta, University of Western Ontario, London, even patients with advanced fibrosis or cirrhosis were able to achieve SVR rates of approximately 62% (Figure 1). “This is vastly different from our previous standard.” According to Yoshida et al., among the 47 Canadian patients in ADVANCE, SVR rates were 85% with 12 weeks of telaprevir vs. 47% with PR (abstract 319).
Similarly, as reported in the SPRINT-2 trial (Poordad et al. N Engl J Med 2011;364:1195-206), which evaluated 1097 patients with HCV genotype 1, those in the boceprevir treatment arms had improved SVR rates (63% and 66% vs. 38% for PR). Patients without cirrhosis were about twice as likely as those with cirrhosis to achieve an SVR.
Figure 1.
Response-guided Therapy
“Shorter time to aviremia turns out to be the single most important predictor of SVR,” remarked Dr. Stephen Shafran, University of Alberta, Edmonton. While dual therapy is associated with a gradual decline in viral load, the addition of a PI produces a “precipitous” drop, he observed. “These are really potent additions in terms of antiviral activity.”
In the ADVANCE trial, the study subjects’ mean HCV RNA level at the start of treatment was 6 log10. “Within a matter of a week or 2 on triple therapy, viral load drops several logs toward undetectability, [confirming] the rapidity of response vs. the control group of double therapy,” Dr. Marotta indicated (Figure 2). The early and sustained clearance of HCV permits patients to receive a shortened course of PR treatment. This treatment model is known as response-guided therapy (RGT). The prospect of a shorter treatment course is appealing for both patients and physicians, Dr. Myers stated. “You shorten the exposure to side effects, for one thing. There is also the aspect of cost; these treatments are expensive.”
Figure 2.
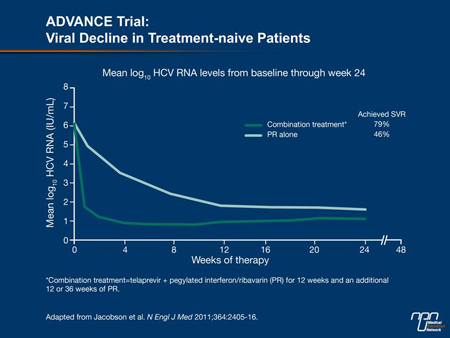
In the ADVANCE study, 58% of patients fulfilled the RGT criteria of undetectable virus at week 4 and week 12 (i.e. at completion of telaprevir administration). For these individuals, subsequent PR therapy was 12 weeks, for a total treatment duration of 24 weeks. Data from ADVANCE and the subsequent ILLUMINATE study (Sherman et al. N Engl J Med 2011;365:1014-24) confirmed that a shortened treatment course did not negatively influence SVR rates. In the SPRINT-2 trial of boceprevir, 44% of patients were eligible for RGT and the SVR rate in this group was 96%, similar to that in patients who received 44 weeks of triple therapy. RGT is not recommended for patients with cirrhosis (F4 fibrosis).
Treatment-experienced Patients
A patient’s previous response to PR reflects the likelihood of response to one of the new triple-therapy regimens, observed Dr. Jordan Feld, University of Toronto, Ontario. In the REALIZE trial of telaprevir (Zeuzem et al. N Engl J Med 2011;364:2417-28), SVR was significantly more likely in patients who experienced disease relapse after a response to dual therapy (86%) than it was in partial responders (57%) or null responders to PR (31%). In the RESPOND-2 trial of boceprevir (Bacon et al. N Engl J Med 2011;354:1207-17), SVR was achieved by 75% of patients with prior relapse and 52% of those with a prior partial response to PR; null responders were not evaluated in this study.
In fact, SVR rates in relapsing patients are higher than those of treatment-naive individuals, Dr. Marotta remarked, “presumably because they have experienced treatment, they are a little more compliant, they are prepared for the side effects, they have tolerated therapy in the past.” The 31% SVR achieved with telaprevir in previous null responders is still noteworthy, he added, because “this is a group that really had no response to double therapy in the past.” Triple therapy with boceprevir has also been shown to lead to significant improvements in SVR in pretreated populations, with relapsing patients faring better than partial responders to previous dual therapy.
Viral load also decreased more markedly in relapsing patients, such that patients with relapsing HCV infection but without cirrhosis are often able to undergo RGT. In a phase II trial of telaprevir, a 24-week total course of therapy (12 with telaprevir) led to SVR in 94% of 52 patients enrolled. This result was comparable to the 95% SVR achieved by relapsers who received a full 48-week course of therapy in the REALIZE trial. Patients with a prior partial or null response are best served with a full course of therapy, the speakers agreed.
As is the case with treatment-naive patients, fibrosis in treatment-experienced individuals blunts the response to either triple therapy regimen in patients with a prior partial or a null response to PR. The treatment of patients with cirrhosis remains challenging, stressed Dr. Morris Sherman, University of Toronto. An open question is whether cirrhotic patients with a previous null response merit further treatment, as these individuals appear the least likely to achieve SVR. In REALIZE, the rate in this population was 14% although, Dr. Sherman cautioned, there were few patients in this category.
Futility Rule
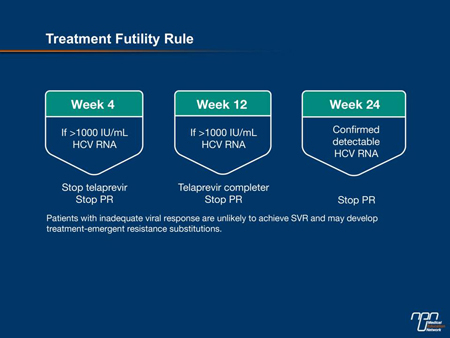
Trial data accumulated to date provide guidance on withdrawal of treatment for lack of efficacy. For triple therapy including telaprevir, for example, patients with an HCV RNA >1000 IU/mL (>3 log10) after 4 weeks should stop all therapies (Figure 3). “[Among patients] who had a viral load >1000 at week 4, there has not been a single SVR... So it is a very robust rule,” affirmed Dr. Marotta. He added that while it may be tempting to continue therapy if the HCV RNA is slightly over that mark, the futility rule should still be applied. “The viral load has already dropped to near zero in the first week or 2. If, at week 4, you see a value that is 1500 or 1200, that viral load is on its way up, not on its way down.”
Figure 3.
Addressing Adverse Effects
Information collected by European researchers indicates that among patients with HCV and cirrhosis receiving triple therapy, adverse events were more common than was reported in clinical trials, several speakers observed.
Anemia is a prominent side effect of the new PIs. “Expect and react to anemia early,” Dr. Marotta recommended. Blood monitoring should be performed regularly in patients receiving these medications, as a 3- to 4-g decrease in hemoglobin may occur very rapidly. The first step in management is to decrease (usually, halve to 600 mg) the patient’s dose of ribavirin. According to Dr. Myers, there is evidence that ribavirin doses in triple therapy with telaprevir can be cut to as low as 200 mg without a negative impact on SVR rates. “Our old lessons about keeping the ribavirin dose maximized really start to be less important when you add in telaprevir and that’s really important for getting these patients through therapy. Never reduce the dose of the PI because that leads to the emergence of resistance,” advised Dr. Feld. If ribavirin dose reduction does not adequately address the anemia, erythropoietin and blood transfusions may be required. Interestingly, anemia is a predictor of SVR, several speakers remarked.
Current data suggest about half of patients receiving telaprevir will experience a rash. Most cases are mild to moderate and can be managed with antihistamines or topical steroids. A severe rash—one that is generalized, characterized by vesicles or bullae or involves mucous membranes—has been observed in 4.8% of patients in phase II and III studies of this agent, compared with 0.4% of those receiving PR alone. Severe rash necessitates telaprevir cessation and a dermatologic consultation. If the problem does not resolve after withdrawal of the PI, it may be necessary to stop ribavirin and pegylated interferon sequentially, stated Dr. Feld. Anorectal signs and symptoms such as pruritus and burning have also been reported by patients receiving telaprevir and may be managed with topical therapies.
Dysgeusia has been a common complaint among patients receiving boceprevir. According to hepatologist Dr. Edward Tam, Vancouver, British Columbia, some patients have been able to reduce this side effect by taking the medication with citrus fruit, banana, ginger or cucumber water.
Given that the new PIs are largely metabolized through the P450 CYP3A4 pathway, drug-drug interactions are possible. Clinicians are advised to discuss possible interactions with a pharmacist or consult advisory Web sites such as www.hep-druginteractions.org/ or www.drug-interactions.com.
Summary
At present, clinicians will likely base their choice of PI on dosing considerations, treatment duration, side-effect profile and personal experience and preference, Dr. Myers indicated. The shorter treatment course of telaprevir may be an advantage, Dr. Sherman remarked.
Treating HCV infection remains challenging in Canada, in part because many patients have inadequate access to medical care and compliance is difficult to achieve. “Up to 2% of the population has hepatitis C but those patients are not coming to our clinics. That’s the funnel everyone has to come through. If we can get people to the funnel, we can cure a lot of people, save health care dollars, and downstream we’ll prevent cirrhosis, transplants and tumours,” Dr. Marotta emphasized.
Triple therapy provides an enormous step toward virologic cure, and additional antiviral agents now in development should also help eradicate the disease and its burden, stated Dr. Marc Bilodeau, Université de Montréal, Quebec. “Another consequence of achieving SVR is that you decrease, potentially, stigmatization of your patients. A patient who is cured of hepatitis C no longer has the heavy burden of infection... And when we treat this disease we also have the opportunity to improve the overall health of these patients… to achieve some important interventions that will have the potential to improve their general medical, psychological and social health.”