Reports
Options for Extending Survival in Advanced Colorectal Cancer: Increasing Evidence for a Chronic Therapy Approach
This report is based on medical evidence presented at sanctioned medical congress, from peer reviewed literature or opinion provided by a qualified healthcare practitioner. The consumption of the information contained within this report is intended for qualified Canadian healthcare practitioners only.
PHYSICIAN PERSPECTIVE - Viewpoint based on literature review
December 2012
Reviewed and Edited by:
Sharlene Gill, MD, MPH, FACP, FRCPC
Gastrointestinal Medical Oncologist
BC Cancer Agency
Associate Professor of Medicine
University of British Columbia
Vancouver, British Columbia
Introduction
Monoclonal antibodies (MAbs) have shifted the opportunities for extended control in the treatment of metastatic colorectal cancer (mCRC). Unlike the direct reduction in disease burden provided by cytotoxic therapies, targeted MAbs can inhibit the molecular events that drive tumour vascularization and proliferation. The potential to provide long-term control of tumour growth by inhibiting these events is continuing to be pursued with the testing of novel regimens. Recently, a phase III trial provided evidence that continuation of the MAb bevacizumab yields a survival benefit even after signs of progression. Given the lower burden of adverse events associated with targeted therapies, this extension of survival represents a meaningful advance. New clinical data with aflibercept and regorafenib, both of which are now available for the treatment of mCRC in some countries, highlight a broader effort to strengthen the armamentarium for the treatment of advanced CRC through targeted treatments.
Metastatic colorectal cancer (mCRC) remains largely an incurable disease but the duration of disease control has been improving since a trial almost 20 years ago when 5-fluorouracil was found to double the median overall survival (OS) relative to supportive care (11 vs. 5 months; P=0.006).1 Other chemotherapies active in mCRC, such as oxaliplatin, irinotecan and capecitabine, have contributed to incremental OS improvements. Progress is now being made with monoclonal antibodies (MAbs) targeted at specific molecular pathways involved in tumour proliferation and angiogenesis. Current first-line regimens, such as FOLFOX, FOLFIRI and XELOX, are the standard chemotherapies to which the addition of targeted therapies have been tested.
Value of Targeted Therapies
In an initial phase III trial with bevacizumab, a humanized MAb directed at vascular endothelial growth factor (VEGF), the median OS among previously untreated patients with mCRC increased from 15.6 to 20.3 months (P<0.001) when this agent was added to the combination of irinotecan, bolus 5-FU and leucovorin (Figure 1).2 Initial trials with cetuximab, a chimeric MAb targeting the epidermal growth factor receptor (EGFR), were performed in patients previously treated for mCRC. In a first line therapy trial, the addition of cetuximab with FOLFIRI chemotherapy resulted in significant improvements in OS (median, 23.5 vs. 20.0 months; hazard ratio [HR] 0.796; P=0.0093), progression-free survival (PFS) (median 9.9 vs. 8.4 months; HR 0.696; P=0.0012) and response (rate 57.3% vs. 39.7%; odds ratio, 2.069; P<0.001) compared with FOLFIRI alone, but this benefit was limited to patients with wild-type KRAS disease.3 No benefit was seen in patients with mutant KRAS disease which represents approximately 40% of all patients with mCRC.4
For second-line therapy and beyond, bevacizumab and the EGFR inhibitors cetuximab and panitumumab have all demonstrated activity in various strategies, including, in some cases, as a monotherapy. In a second-line trial conducted in patients previously treated with fluoropyrimidine and irinotecan, the median OS increased from 10.8 to 12.9 months (P=0.0011) with the addition of bevacizumab relative to FOLFOX alone.5 In the NCIC.CO17 trial with cetuximab conducted in patients previously treated with fluoropyrimidine, irinotecan and oxaliplatin, the median OS increased from 4.6 to 6.1 months (P=0.005) on cetuximab monotherapy relative to supportive care.6 In an analysis of the subset of patients with wild-type KRAS disease, the median OS increased from 4.8 to 9.5 months (P<0.001).7 Panitumumab monotherapy was studied in a similar population of patients who progressed on standard chemotherapy. While a significant OS benefit was not observed in this cross-over study, the improvement in PFS did reach statistical significance (P<0.0001).8 Like cetuximab, benefit with panitumumab has been limited to those with KRAS wild-type tumours.9
Figure 1.
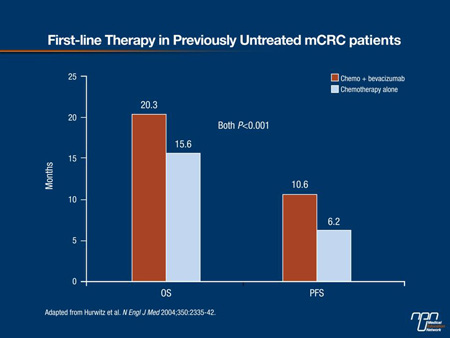
Treatment guidelines, reflecting these results, now include MAbs in both first- and second-line therapies.10 Importantly, the value of these targeted therapies, which suppress tumour growth rather than kill tumour cells, may not be fully reflected in traditional outcomes. In extending survival while preserving an adequate quality of life, the activity of these agents has introduced the potential for a prolonged, chronic management strategy even in the absence of a cure. Support for a chronic therapy approach in mCRC is highlighted by the results from trials reported within the last year.
Maintenance after First Progression
The newly published data from the bevacizumab ML18147 randomized phase III trial represents the latest step toward prolonged disease control with continued anti-VEGF therapy.9 In this open-label trial, patients with progressive mCRC after first-line bevacizumab with chemotherapy were randomized to receive second-line chemotherapy with or without continuation of bevacizumab.
The ML18147 study randomized 820 patients at 220 treatment centres. Eligibility included unresectable mCRC, previous treatment with a standard first-line therapy that included a fluoropyrimidine plus oxaliplatin or irinotecan, and progression within 3 months of discontinuing the first-line regimen. Patients with early progression (within 3 months of starting first-line therapy) were excluded. The second-line chemotherapy was based on the first-line exposure so that those receiving first-line oxaliplatin received irinotecan second-line or the reverse.
The advantage of VEGF inhibition for the primary end point of median OS was highly significant, producing a hazard ratio (HR) of 0.81 (95% CI, 0.69-0.94; P=0.0062). In absolute terms, the median OS from start of 2nd line therapy improved from 9.8 months to 11.2 months with the addition of bevacizumab (Figure 2). The median PFS was 5.7 months for those randomized to bevacizumab vs. 4.1 months for those on chemotherapy alone (HR 0.68; P<0.0001). Independent of chemotherapy regimen, the advantage of bevacizumab remained largely consistent across prespecified subgroups defined by ECOG status, age and number of organs with metastases.
Figure 2. ML18147 study: Second-line Bevacizumab
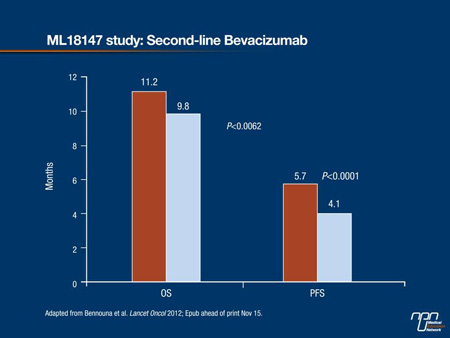
The types and severities of adverse events were similar in the 2 study arms. Although the absolute proportion of patients with a grade ≥3 adverse event was greater on bevacizumab (64% vs. 57%), the differences for any specific event were modest. For example, both of the 2 most common grade ≥3 adverse events, neutropenia (16% vs. 13%) and diarrhea (10% vs. 8%), were slightly more common on bevacizumab, but no other grade ≥3 adverse event was observed in >6% of either group. Of 7 deaths considered to be treatment related, 4 occurred in the bevacizumab group and 3 in the chemotherapy alone group.
These data confirm previous observational data, such as those generated by the BRiTE study,11 which have documented benefit from maintenance bevacizumab in patients receiving more than one course of therapy. Unlike cytotoxic regimens to which tumours typically develop diminished response after an initial course, the ML18147 results indicate that resistance develops less readily to VEGF inhibition.
Additional support for the value of maintenance VEGF inhibition was produced in the unpublished BEBYP trial presented as a latebreaker at the most recent European Society for Medical Oncology (ESMO).12 Conducted by the Gruppo Oncologico Nord Ovest (GONO), the trial had a similar design to ML18147 and was stopped prematurely when results of ML18147 became available.
At the time that the trial was halted, 185 patients with mCRC who had received bevacizumab with first-line chemotherapy were randomized to receive a second-line chemotherapy ±bevacizumab. Although the study had limited follow-up due to the early termination to demonstrate a difference in OS, the PFS advantage was statistically significant with a HR of 0.65 (95% CI, 0.48-0.89; P=0.0062). In absolute terms, the median PFS climbed from 4.97 months to 6.77 months. Again, the benefit was similar across prespecified subgroups.
Chronic Control of mCRC: A Paradigm Shift
The results of these recent studies are clinically relevant and they have important implications for the future of mCRC treatment because they intensify interest in the possibility of sustained control of unresectable cancers when cure is an unlikely outcome. The benefit of persistent inhibition of VEGF across first- and second-line therapies may be relevant to other treatments targeted at VEGF, such as aflibercept, which blocks binding of both VEGF-A and VEGF-B as well as placental growth factor,13 or other molecular pathways, such as EGFR or the enzymes that govern intracellular proliferation signalling cascades.
The potential for initiating patients on a standard chemotherapy regimen with one or more molecular pathway inhibitors and then transitioning to alternative strategies is encouraged by the availability of new agents. Aflibercept was recently approved by the FDA on the basis of the phase III VELOUR trial which associated this therapy with a mean 1.4 month improvement in OS when combined with second-line FOLFIRI in patients previously treated with FOLFOX.14 Of the 1226 patients randomized, 373 (30%) also had previous exposure to bevacizumab. The improvements in both OS and PFS were similar regardless of prior bevacizumab use.15
Regorafenib, also recently approved by the FDA, is the first oral, small-molecule multikinase inhibitor to be made available for the treatment of mCRC. Acting on tyrosine kinases that participate in the signalling cascade for VEGF and EGFR, regorafenib demonstrated benefit in a phase III study of patients with mCRC who progressed after standard therapies, including 5FU, irinotecan, oxaliplatin, bevacizumab, cetuximab and panitumumab (Figure 3).16 In this population, oral regorafenib increased the median OS to 6.4 months from the 5.0 months observed in those receiving supportive care alone (HR 0.77, 95% CI, 0.64-0.94; P=0.0052). The most common grade ≥3 adverse event was hand-foot skin reactions (17%), but no other grade ≥3 side effect occurred in more than 10% of patients.
Figure 3:
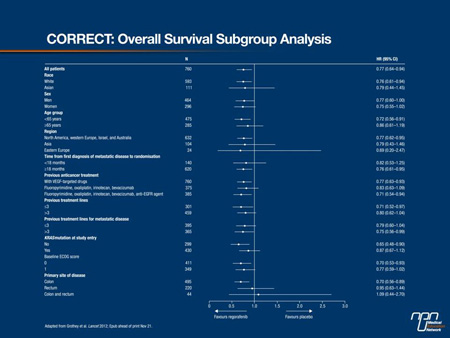
The tolerability of these targeted therapies represents an important dimension of the principle that mCRC might be increasingly managed as a chronic disease with the goal of a sustained survival and preserved quality of life. Although cure remains elusive for the majority of patients with mCRC, these recent advances are important steps towards continuing to improve both quantity and quality of life.
Conclusion
By demonstrating continued benefit from bevacizumab in both first- and second-line therapy, the phase III ML 18147 trial has implications for redefining the management of unresectable mCRC. It extends evidence that continued blockage of the molecular mechanisms of tumour proliferation and angiogenesis can extend survival. The introduction of aflibercept and regorafenib on the basis of favourable activity in advanced mCRC further encourages the potential for additional survival advances as the optimal strategies for the use of targeted therapies are defined.
References
1. Scheithauer et al. Randomised comparison of combination chemotherapy plus supportive care with supportive care alone in patients with metastatic colorectal cancer. BMJ 1993;306(6880):752-5.
2. Hurwitz et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004;350(23):2335-42.
3. Van Cutsem et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med 2009;360(14):1408-17.
4. Van Cutsem et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status.
J Clin Oncol 2011;29(15):2011-19.
5. Giantonio et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol 2007;25(12):1539-44.
6. Jonker et al. Cetuximab for the treatment of colorectal cancer. N Engl J Med 2007;357(20):2040-8.
7. Karapetis et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med 2008;359(17):1757-65.
8. Van Cutsem et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol 2007;25(13):1658-64.
9. Amado et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol 2008;26(10):1626-34.
10. Edwards et al. A systematic review of treatment guidelines for metastatic colorectal cancer. Colorectal Dis 2012;14(2):e31-e47.
11. Grothey et al. Bevacizumab beyond first progression is associated with prolonged overall survival in metastatic colorectal cancer: results from a large observational cohort study (BRiTE). J Clin Oncol 2008;26(33):5326-34.
12. Masi et al. A randomized phase III study evaluating the continuation of bevacizumab (BV) beyond progression in metastatic colorectal cancer patients who received BV as part of first-line treatment: results of the BEBYP trial by the Gruppo Oncologico Nord Ovest (GONO). European Society for Medical Oncology (ESMO) 2012; Vienna, Austria. Abstract LBA17.
13. Mitchell EP. Targeted Therapy for Metastatic Colorectal Cancer: Role of Aflibercept. Clin Colorectal Cancer 2012;Epub ahead of print Oct 24.
14. Joulain et al. Aflibercept versus placebo in combination with FOLFIRI in previously treated metastatic colorectal cancer: mean overall survival estimation from a phase III
trial (VELOUR). American Society of Clinical Oncology (ASCO) 2012; Chicago. Abstract 3602.
15. Allegra et al. Effects of prior bevacizumab use on outcomes from the VELOUR study: a phase III study of aflibercept and FOLFIRI in patients with metastatic colorectal cancer after failure of an oxaliplatin regimen. In: American Society of Clinical Oncology (ASCO) 2012; Chicago. Abstract 3605.
16. Grothey et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2012;Epub ahead of print Nov 22.