Reports
Pulmonary Hypertension: New Therapies in Chronic Thromboembolic Pulmonary Hypertension and Pulmonary Arterial Hypertension
This report is based on medical evidence presented at sanctioned medical congress, from peer reviewed literature or opinion provided by a qualified healthcare practitioner. The consumption of the information contained within this report is intended for qualified Canadian healthcare practitioners only.
ABSTRACTS in PERSPECTIVE - Based on presentations from the Annual Congress of the European Respiratory Society (ERS)
Barcelona, Spain / September 7-11, 2013
EDITORIAL OVERVIEW:
John Granton, MD
Director, Pulmonary Hypertension Program
Toronto General Hospital
University of Toronto
Toronto, Ontario
John Swiston, MD
Director, Pulmonary Hypertension Program
Vancouver General Hospital
University of British Columbia
Vancouver, British Columbia
Positive outcomes from recently completed Phase III clinical trials promise to expand opportunities to manage pulmonary hypertension (PH). Detailed analyses of these Phase III trials were presented at the ERS 2013 along with additional data relevant to current efforts to improve care for this condition. The Phase III trials were conducted with two novel agents: riociguat, a stimulator of soluble guanylate cyclase, a key component of the nitric oxide signaling pathway, and macitentan, a novel agent that demonstrates efficacy in an existing target, the endothelin pathway. Due to the complexity of PH and its broad array of etiologies, the expansion of treatment options has the potential to provide additional strategies to better manage PH and address some of the limitations of current endothelin-receptor antagonists. Some of the limitations in the design and end points of previous clinical trials were also addressed in the study evaluating the effect of macitentan.
Pulmonary Hypertension:
Differentiating Subtypes to Improve Management
Pulmonary hypertension (PH) defines a group of disorders associated with an elevation in mean pulmonary arterial pressure >25 mmHg and has been classified into 5 different categories (Simmoneau J Am Coll Cardiol 2009;54:S43-54). The first category, pulmonary arterial hypertension (PAH), is defined by a mean pulmonary artery pressure of ≥25 mmHg in the absence of left-sided heart disease and is characterized by progressive right-sided heart failure (Badesch et al. Chest 2007;131:1917-28). PAH may be idiopathic or heritable or associated with other conditions such as connective tissue disease, congenital cardiac lesions, anorexigens, HIV and portal hypertension. Of the four other categories of PH recognized by the World Health Organization (WHO) all are capable of producing signs and symptoms that resemble PAH. Distinguishing between these forms, which in addition to PAH and a category of miscellaneous etiologies includes PH due to left heart disease (PHLD-WHO Group 2), PH due to lung diseases or hypoxia (PHLDH-WHO Group 3), and chronic thromboembolic pulmonary hypertension (CTEPH-WHO Group 4), is essential for evaluating prognosis and directing appropriate therapy (Galiè et al. Eur Heart J 2009;30:2493-537).
Although prevalence rates of PH have been variably estimated, all forms of this condition are uncommon. In a Scottish registry, the annual incidence and overall prevalence were estimated at 7.6 cases and 26 cases per million, respectively (Peacock et al. Eur Respir J 2007;30:104-9). An analysis of a national database in France placed these figures, respectively, at 2.4 and 15 cases per million. In one registry, idiopathic PAH accounted for 40% of PAH cases (Humbert et al. Am J Respir Crit Care Med 2006;173:1023-30), suggesting the prevalence of WHO Groups 2 - 4 is even lower. However, it is acknowledged that CTEPH is one of the most common causes of severe PH (Hoeper et al. Circulation 2006;113:2011-20).
New Therapeutic Options in PH
PH, in most cases, is a progressive condition that causes right-sided heart failure and is associated with significant morbidity and mortality. Although the rate of progression varies substantially (Galiè et al. Eur Heart J 2009;30:2493-537), resolution of PH is uncommon once tissue damage is incurred. The most conspicuous exception is CTEPH for which thromboendarterectomy has curative potential in the subset of patients with resectable lesions, no contraindications for surgery, and timely access to centers where such procedures are performed (Fedello et al. N Engl J Med 2001;345:1465-72). Due to the complexity of PH, the frequency of co-morbid conditions, and the impact of the PH on broad aspects of daily living, guidelines strongly recommend a multidisciplinary approach to care along with pharmacologic therapy to control symptoms and improve quality of life (Badesch et al. Chest 2007;131:1917-28; Galiè et al. Eur Heart J 2009;30:2493-537).
In patients with PAH, the treatment currently includes prostanoids, endothelin-receptor antagonists (ERA), phosphodiesterase type 5 inhibitors, and calcium channel blockers (McLaughlin et al. J Am Coll Cardiol 2009;53:1573-1619). All are associated with increased exercise capacity on such measures as the 6-minute walk distance (6MWD) test. Since their introduction, survival in patients with PH has improved (Galiè et al. Eur Heart J 2009;30:394-403), but the relative benefit of these therapies for preventing vascular remodeling or altering the natural history of disease has not been studied in a controlled fashion in comparative trials.
At the ERS 2013, new data from three Phase III trials published just weeks earlier, provided a framework to reconsider pharmacologic management of PH. Two of the studies were conducted with riociguat, a stimulator of soluble guanylate cyclase (sGC), a novel therapeutic target in PH. The third trial was conducted with macitentan, a unique, potent ERA. Both the PATENT-1 trial with riociguat (Ghofrani et al. N Engl J Med 2013;369:330-40) and the SERAPHIN trial with macitentan (Pulido et al. N Engl J Med 2013;369:809-18) were conducted in patients with PAH. The CHEST-1 trial with riociguat was conducted in patients with CTEPH (Ghofrani et al. N Engl J Med 2013;369:319-29).
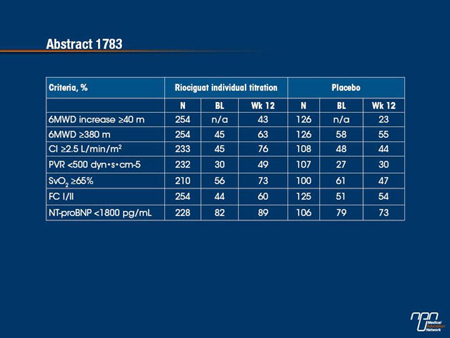
The PATENT-1 study, which randomized 443 patients, reported that riociguat relative to placebo led to a significant improvement in the primary end point of 6MWD (P<0.001). At the ERS, a responder analysis provided additional support for the evidence of efficacy by demonstrating a greater increase in the proportion of riociguat versus placebo patients meeting favourable prognostic criteria (increase in 6MWD ≥40 m, 6MWD ≥380 m, cardiac index ≥2.5 L/min/m2, PVR <500 dyn•s•cm-5, NT-proBNP <1800 pg/mL) (Grunig et al. ERS 2013, Abstract 1783). While the proportions meeting these criteria were similar in both groups at baseline, at 12 weeks, the proportions increased in those receiving riociguat but declined or remained the same in those receiving placebo.
These improvements are consistent with the activity of sGC, a natural receptor for nitric oxide (NO). In animal models sGC has been shown to increase synthesis of cyclic guanosine monophosphate (cGMP), which controls vascular tone, prevents smooth muscle cell proliferation, and demonstrates both anti-fibrotic and anti-inflammatory properties (Schermuly et al. Eur Respir J 2008;32:881-91). The favourable clinical effects of riociguat are attributed to restoration of a more physiologic NO-sGC-cGMP signaling axis.
The validity of stimulating sGC as a therapeutic target in PH was further demonstrated in the CHEST-1 trial, which associated riociguat with significant improvements in exercise capacity relative to placebo in patients considered to have inoperable or persistent/recurrent CTEPH, essentially an orphaned form of PH for which there are no approved pharmacologic therapies. In CHEST-1, which randomized 261 patients, riociguat led to a significant improvement on the primary end point by increasing 6MWD (P<0.001) relative to placebo. Riociguat was well tolerated and is currently licensed in Canada for the treatment of inoperable or persistent/recurrent CTEPH. It is worth emphasizing that surgical therapy is still the treatment of choice for many patients and that available pharmacotherapy should not delay or replace surgical treatment of this condition.
New data from CHEST-1 presented at the ERS reinforced the primary findings seen in this study. Similar to the responder analysis performed with the PATENT-1 data, riociguat was associated with improvement in other prognostically relevant secondary end points, including an improvement in WHO functional class (FC) (Armini et al. ERS 2013, Abstract 2598). Importantly, data presented from CHEST-2, (an open-label extension of CHEST-1) showed sustained functional improvements in FC after one year on therapy (Simmoneau et al. ERS 2013, Abstract 1785).
While the PATENT and CHEST trials provide mutually supportive evidence for sGC stimulation in the treatment of PH and represent the first therapy to demonstrate efficacy and safety across two forms of PH, the results of the Phase III trial with macitentan reinforce the value of existing targets for treating PAH. Macitentan is distinguished from existing ERA agents by enhanced tissue penetration and sustained receptor binding (Gatfield et al. PLoS One 2012;7:e47662). In addition to evaluating a novel ERA, the SERAPHIN trial is considered important because it is the first Phase III trial in PH to be driven by clinical events and employ a standardized composite end point of events, including mortality. At the ERS 2013, new data from SERAPHIN described an improvement in cardiopulmonary hemodynamics with macitentan relative to placebo (Sitbon et al. ERS 2013, Abstract 4060) and confirmed that the improvements in the composite end point evaluating morbidity and mortality were achieved with a low risk of adverse events (Ghofrani et al. ERS 2013, Abstract 1786).
The advances in therapy highlight the importance of early diagnosis and determination of the cause of PH. In this context, several studies presented at the ERS are of interest, including a study demonstrating that a simple questionnaire may be effective for identifying CTEPH in patients who have had a pulmonary embolism (PE) (Held et al. ERS 2013, Abstract 2602). In this study of 123 patients with recent PE who responded to a simple follow-up telephone questionnaire, 10 cases of CTEPH, of which 3 were negative on echocardiography were found. In another study, the presence of fibrosis and emphysema in PH patients was associated with worse hemodynamics and right ventricular function relative to chronic obstructive pulmonary disease (COPD)-associated PH or PH associated with interstitial lung disease. In these patients, the identification of new drugs with novel mechanisms of action provides new opportunities for clinical studies.
Conclusion
In most individuals, PH is a progressive disorder for which mortality rates remain high despite advances in treatment (Archer et al. Circulation 2010;121:2045-66). The publication of three positive Phase III clinical trials within weeks of each other, reinforced by data presented at the ERS, holds the promise of new opportunities to improve outcomes in these patients. Of these, an event-driven trial with the novel ERA macitentan has demonstrated results that evaluated patient relevant outcomes, rather than simply exercise capacity as assessed by the 6MWD. The two studies of riociguat confirm the viability of sGC as a new therapeutic target in PAH, and for the first time, demonstrate benefit in patients with inoperable or persistent/recurrent CTEPH. Together, these data suggest meaningful clinical progress in a set of difficult disorders.
ABSTRACT 1783
Riociguat for the treatment of pulmonary arterial hypertension (PAH): A responder analysis from the phase III PATENT-1 study
E. Grünig, N. Galiè, M. Humbert, A. M. Keogh, D. Langleben, L. J. Rubin, R. Speich, A. Fritsch, N. Davie, H. A. Ghofrani (Heidelberg, Wuppertal, Giessen, Germany; Bologna, Italy; Le Kremlin-Bicêtre, France; Sydney, Australia; Montreal, Canada; La Jolla, United States Of America; Zurich, Switzerland)
Bakground: In PATENT-1, riociguat significantly improved 6-min walking distance (6MWD) and a range of secondary endpoints, including hemodynamics, NT-proBNP, and WHO functional class (FC), in patients (pts) with PAH. For several of these endpoints, threshold criteria have been defined that correlate with favorable clinical outcome.
Aim: To investigate the proportion of pts who fulfilled these criteria in PATENT-1.
Methods: PATENT-1 was a double-blind randomized trial in which pts with PAH received 12 wks’ oral treatment with placebo, an individual titration of riociguat (up to 2.5 mg tid), or a capped titration of riociguat (up to 1.5 mg tid). Increase in 6MWD ≥40 m, 6MWD ≥380 m, cardiac index (CI) ≥2.5 L/min/m2, PVR <500 dyn•s•cm-5, mixed venous oxygen saturation (SvO2) ≥65%, FC I/II, and NT-proBNP <1800 pg/mL were chosen as criteria of a positive response based on studies showing their prognostic relevance at baseline (BL) and after targeted therapy.
Results: Similar proportions of pts met the selected criteria in the riociguat and placebo groups at baseline. The proportion of pts who met these criteria at Wk 12 was increased in the riociguat group, while it remained unchanged or decreased in the placebo group.
Conclusions: Riociguat increased the proportion of pts who fulfilled criteria defining a positive response to therapy.
Commentary on abstract 1783
Secondary end points from major Phase III trials provide an opportunity to evaluate efficacy in a broader context. The primary end point of the PATENT-1 study, which evaluated the sCC stimulator riociguat in pulmonary arterial hypertension (PAH), was a demonstration of an improvement in exercise capacity. In this analysis, meaningful thresholds of effect were evaluated for a variety of additional clinical and hemodynamic outcomes for those randomized to riociguat relative to those randomized to placebo. When compared to baseline, the proportion of patients meeting each of these criteria increased in the riociguat group, but remained the same or decreased in the placebo group. The substantial increases observed in the hemodynamic parameters, such as increased cardiac index and reduced pulmonary vascular resistance, provide reassurance that riociguat is active in addressing the underlying pathophysiology.
Questions and answers with Dr. John Swiston and Dr. John Granton
Q: How does this data potentially impact your practice going forward?
A: Dr. Swiston: Secondary analyses in randomized clinical trials allow for further exploration of the effect of the study medication. It is reassuring to see that the secondary end points and exploratory analysis support the conclusion of the primary end point. The data presented in this abstract lend support to the primary outcome of the PATENT-1 study and increase my confidence in the efficacy of riociguat in patients with PAH.
A: Dr. Granton: Many centers have adopted a "treat to target" philosophy of care for patients with PAH. Although this is an exploratory analysis, this finding aligns with how we actually practice. Therefore these results may resonate with practicing clinicians.
ABSTRACT 2599
Pulmonary endarterectomy for chronic thromboembolic pulmonary hypertension with distal lesions
A. M. D’Armini, M. Morsolini, G. Mattiucci, V. Grazioli, N. Vistarini, R. Dore (Pavia, Italy)
Background: As there are no well-defined criteria to discriminate proximal from distal obstructive lesions in chronic thromboembolic pulmonary hypertension (CTEPH), the operability assessment for pulmonary endarterectomy (PEA) remains the major concern.
Aim: The intraoperative classification of CTEPH describes different types of arterial obstruction, based on anatomy and location. We describe our experience with the more distal disease (type 3).
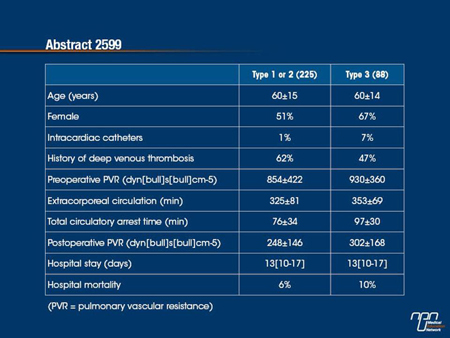
Methods: From 1994, 458 PEAs were performed at our center. From 2005 onward, the operability assessments and the operations were carried out by one surgeon. Into the cohort of 313 consecutive patients (pts) operated from 2005 to 2012, 88 (28%) presented with type 3 CTEPH, and 225 (72%) with type 1 or 2.
Results: The comparison between the 2 groups is shown in table. Thirty-three (37.5%) pts with type 3 disease were younger than 60 years and were severely symptomatic, and would otherwise have been included in lung transplantation (LTx) waiting list.
Conclusion: In experienced centers, PEA is a successful and safe operation even when performed in pts presenting with distal CTEPH. Pts should not be considered inoperable unless they have been referred to an experienced surgeon, as they could benefit from conservative surgery instead of LTx.
Commentary on abstract 2599
In patients with chronic thromboembolic pulmonary hypertension (CTEPH), pulmonary endarterectomy is generally performed only in those with proximal disease, whether defined as fresh thrombus in the main lobar pulmonary artery (type 1) or intimal thickening and fibrosis in a proximal segmental artery (type 2). Distal thrombi, including segmental lesions (type 3), are considered more challenging and sometimes characterized as inoperable. In this analysis, PEA outcomes were compared for proximal (type 1 and 2) and distal (type 3) lesions at a single centre evaluating consecutive patients. The authors considered outcomes in the distal group to be acceptable even though mortality (10% vs. 6%) and the average level of pulmonary vascular resistance (PVR) following surgery were higher in the distal group. The authors conclude that inoperability may be a relative term dependent on surgical skills.
Questions and answers with Dr. John Swiston and Dr. John Granton
Q: Can you comment on your institution’s experience with distal lesions. What is the success rate for these interventions?
A: Dr. Swiston: Patients with chronic thromboembolic disease are referred to an experienced PEA centre for evaluation of candidacy for PEA regardless of the clot anatomy. It is my experience that patients referred with distal lesions are less likely to be considered candidates for PEA and, if they are accepted for surgery, are more likely to have residual PH after their surgery.
A: Dr. Granton: One of the important roles of an experienced centre is to accurately distinguish type 1 and 2 from distal or type 3 disease. The availability of an agent to treat patients with distal disease emphasizes the importance of making this distinction. Whether surgery combined with medical therapy is superior to medical or surgical therapy alone for these patients is not established. It is our practice to only operate on patients with type 1 and 2 disease. We look forward now to being able to offer medical therapy to this other orphaned group of patients.
Q: In situations of distal lesions, where surgery is not an option, how do you treat these patients at your institution?
A: Dr. Swiston: Patients with distal lesions and pulmonary hypertension that are not deemed to be surgically accessible are considered for targeted PH therapy depending on their clinical assessment and disease severity. Currently the therapies considered are ERAs or PDEIs; however, with the results of the CHEST-1 study, I anticipate the use of riociguat in these patients.
A: Dr. Granton: Historically we have treated these patients with PDEI’s or prostanoids. In patients with advanced disease and refractory right-sided heart failure we have offered lung transplantation. It is hoped that the availability of riociguat will benefit these patients with refractory or non-surgical disease.
ABSTRACT 2598
Riociguat for the treatment of inoperable CTEPH or persistent/recurrent PH after pulmonary endarterectomy (PEA): A responder analysis from the phase III CHEST-1 study
A. M. D’Armini, H. A. Ghofrani, N. H. Kim, E. Mayer, G. Simonneau, M. R. Wilkins, T. Pulido, A. Fritsch, N. Davie, M. M. Hoeper (Pavia, Italy; Giessen, Bad Nauheim, Wuppertal, Hannover, Germany; San Diego, United States Of America; Le Kremlin-Bicêtre, France; London, United Kingdom; Mexico City, Mexico)
Background: EA is the treatment of choice for CTEPH, but a proportion of patients (pts) are ineligible or have persistent/recurrent PH after PEA. CHEST-1 showed that riociguat is a promising therapy for such pts, as it improved 6-min walking distance (6MWD), hemodynamics, WHO functional class (FC), and NT-proBNP.
Aims: To determine the proportion of pts in CHEST-1 who achieved responder thresholds shown previously to correlate with improved outcome in pts with other forms of PH, at baseline (BL) and after targeted therapy.
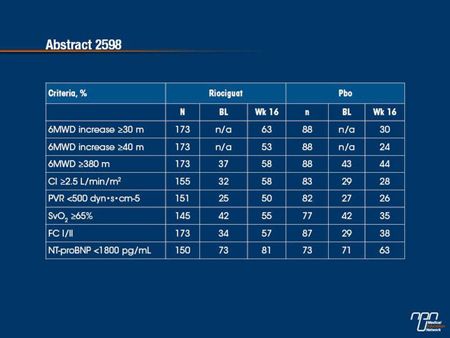
Methods: In this randomized, double-blind, Phase III study, pts received placebo (pbo) or individually titrated riociguat (up to 2.5 mg tid) for 16 wks. The criteria of a positive response were: 6MWD increase ≥30 m and ≥40 m, 6MWD ≥380 m, cardiac index (CI) ≥2.5 L/min/m2, PVR <500 dyn•s•cm-5, SvO2 ≥65%, FC I/II, and NT-proBNP <1800 pg/mL.
Results: The proportion of pts meeting these criteria at BL and Wk 16 is shown below. At BL, the proportions were similar in the pbo and riociguat arms. Riociguat increased the proportion of pts achieving these criteria at Wk 16; minimal improvements or decreases were seen in the pbo arm for most parameters.
Conclusions: Riociguat increased the proportion of CTEPH pts achieving criteria defining a positive response to therapy.
Commentary on abstract 2598
In the CHEST-1 trial, conducted in patients with chronic thromboembolic pulmonary hypertension (CTEPH), the sGC stimulator riociguat was associated with a significant improvement in the primary end point of exercise capacity relative to placebo. In this analysis, the relative effect on secondary outcomes, expressed as the proportion of patients achieving clinically meaningful improvements from baseline, was evaluated. In the riociguat group, all of the measures, which included both clinical and hemodynamic outcomes, were improved relative to baseline at the end of the study period. In the placebo group, there was a modest increase at 16 weeks in the proportion of patients who improved their functional class but all other measured outcomes remained the same or declined. The improvements in hemodynamic parameters, such as pulmonary vascular resistance (PVR) and cardiac index (CI) on riociguat are consistent with the observed improvements in clinical measures.
Questions and answers with Dr. John Swiston and Dr. John Granton
Q: Are these study end points relevant to your current practice?
A: Dr. Swiston: The data presented in this abstract lend support to the primary outcome of the CHEST-1 study and increase my confidence in the efficacy of riociguat in patients with inoperable CTEPH or persistent/recurrent PH after PEA.
A: Dr. Granton: As mentioned earlier, these findings may resonate with many of us who adopt a "treat to target" approach to care. These findings, from an exploratory analysis of the data, offer reassurance that the primary outcome was robust and meaningful.
Q: How do these results compare to other contemporary trials e.g. the BENEFIT trial?
A: Dr. Swiston: Unlike the CHEST-1study, the BENEFIT trial, evaluating the efficacy of bosentan in inoperable CTEPH or persistent/recurrent PH after PEA, did not meet the primary end point of six-minute walk distance. Analysis of secondary end points in this trial did show an improvement in some hemodynamic parameters but not with the consistency that was demonstrated with riociguat in CHEST-1.
A: Dr. Granton: As Dr. Swiston points out, the CHEST-1 study was able to meet its primary end point in this group of patients. Therefore I suspect that most clinicians will turn to riociguat as the primary agent for this group of patients.
ABSTRACT 1785
An interim analysis of the phase III riociguat long-term extension study in CTEPH (CHEST-2)
G. Simonneau, A. M. D’Armini, H. A. Ghofrani, F. Grimminger, M. M. Hoeper, P. Jansa, N. H. Kim, C. Wang, M. R. Wilkins, A. Fritsch, N. Davie, G. Weimann, E. Mayer (Le Kremlin-Bicêtre, France; Pavia, Italy; Giessen, Hannover, Wuppertal, Bad Nauheim, Germany; Prague, Czech Republic; San Diego, United States Of America; Beijing, China; London, United Kingdom)
Background: In the 16-wk CHEST-1 study, riociguat, a novel sGC stimulator, was well tolerated in CTEPH patients (pts) and significantly improved 6-min walking distance (6MWD) and WHO functional class (FC) vs placebo (pbo).
Objectives: The CHEST-2 open-label extension assessed the long-term safety and efficacy of riociguat.
Methods: Pts with inoperable CTEPH or persistent/recurrent PH after pulmonary endarterectomy were eligible to enter CHEST-2 after completion of CHEST-1 without ongoing riociguat-related serious AEs. The primary endpoints were safety and tolerability. Secondary endpoints included change in 6MWD and FC.
Results: Of 233 pts entering CHEST-2, 194 were eligible for this interim analysis (cut-off May 2012; median treatment duration ?1 y). Riociguat was well tolerated during CHEST-2; 1% of pts withdrew due to AEs. CHEST-1 baseline (BL) 6MWD was 351 m in riociguat pts and 365 m in pbo pts, increasing by 51 m and 4 m, respectively at the end of CHEST-1. After 12 wks of CHEST-2, 6MWD increased by 63 m in former riociguat pts and 35 m in former pbo pts, vs CHEST-1 BL. After 1 y (overall cohort; n=93), change in 6MWD was 48 m. The number of pts with FC I/II/III/IV at CHEST-1 BL was 2/44/80/3 in the riociguat arm and 0/20/42/2 in the pbo arm. At the end of CHEST-1, FC was improved/stable/worsened in 35/61/4% of riociguat pts and 14/83/3% of pbo pts vs CHEST-1 BL. After 12 wks of CHEST-2, FC was improved/stable/worsened in 41/56/3% of former riociguat pts and 38/58/5% of former pbo pts, vs CHEST-1 BL. Preliminary data suggest improvement in FC for up to 1 y.
Conclusions: Riociguat has a good long-term safety profile and is the first therapy to show sustained benefits in 6MWD and FC in CTEPH pts.
Commentary on abstract 1785
After completion of CHEST-1, a 16-week Phase III trial that associated riociguat with a significant improvement in exercise capacity, patients in either the riociguat or placebo arms were permitted to enroll in CHEST-2, an open-label extension study testing efficacy and safety over a longer period. Data presented here from CHEST-2 included 194 of the 233 eligible CTEPH patients. After 12 weeks of additional therapy, the 6-minute walk distance (6MWD) was increased by 63 meters in those initially treated with riociguat and by 35 meters in the placebo group. In the subgroup of 93 patients who completed one year of therapy, the average 6MWD increase was 48 meters. There was an increase in the proportion of patients with an improvement in functional class after 12 weeks, with preliminary evidence that this improvement was sustained in some cases at one year. There were no unexpected adverse events, with just 1% of those entering CHEST-2 withdrawing for a side effect. The data support the efficacy and safety of extended riociguat therapy.
Questions and answers with Dr. John Swiston and Dr. John Granton
Q: How do these long-term results help you better understand this potential new therapy?
A: Dr. Swiston: The results of the CHEST-2 extension study are encouraging in that they suggest that the efficacy of riociguat is sustained beyond the relatively short duration of the primary clinical trial.
A: Dr. Granton: These results support the notion that the initial treatment effect is likely robust and sustained. Longer-term data also supports that the agent is safe and provides a more reliable side effect profile. However, as 39 patients (16%) of the initial cohort were not evaluated/reported, the results need to be interpreted with some caution.
ABSTRACT 1781
Efficacy of riociguat in patients with inoperable CTEPH vs. persistent/recurrent PH after pulmonary endarterectomy (PEA): Results from the phase III CHEST-1 study
E. Mayer, A. M. D’Armini, H. A. Ghofrani, E. Grünig, P. Jansa, N. H. Kim, G. Simonneau, A. Torbicki, C. Wang, M. R. Wilkins, N. Davie, A. Fritsch, M. M. Hoeper (Bad Nauheim, Giessen, Heidelberg, Wuppertal, Hannover, Germany; Pavia, Italy; Prague, Czech Republic; San Diego, United States Of America; Le Kremlin-Bicêtre, France; Warsaw, Poland; Beijing, China; London, United Kingdom)
Background: In CHEST-1, riociguat significantly improved several clinically relevant endpoints in CTEPH patients (pts).
Aims: Here we describe and compare its clinical effect in subgroups of pts with inoperable and persistent/recurrent CTEPH.
Methods: Pts (34% male, mean age 59 y) received placebo (pbo) or riociguat (up to 2.5 mg tid) for 16 wks. The primary endpoint was change in 6MWD; secondary endpoints were change in PVR, NT-proBNP, WHO functional class (FC), time to clinical worsening, Borg dyspnea score, and EQ-5D and Living with PH (LPH) questionnaires.
Results: At baseline (BL), inoperable pts had lower 6MWD (341±80 vs 363±76 m) and higher PVR (861±452 vs 595±233 dyn•s•cm-5) and NT-proBNP (1732±2690 vs 1100±1150 pg/mL) vs persistent/recurrent pts. Selected endpoints and safety data are shown below.
Conclusions: Riociguat improved primary and secondary endpoints in both pts with inoperable and pts with persistent/recurrent CTEPH vs BL, with a more marked effect in inoperable pts.

Commentary on abstract 1781
In the CHEST-1 trial, which associated riociguat with improved exercise capacity relative to placebo in patients with chronic thromboembolic pulmonary hypertension (CTEPH), the majority of enrolled patients had inoperable disease, but 28% had recurrent or persistent CTEPH. In this analysis, the goal was to evaluate the relative benefits in these two groups. While patients with inoperable CTEPH had a lower exercise endurance and a higher pulmonary vascular resistance (PVR) at baseline, both groups had substantial improvements on riociguat relative to placebo for both primary and secondary outcomes. However, relative improvements were generally greater in the inoperable subgroup when compared to those with persistent or recurrent CTEPH. This included both the relative increase in 6-minute walk distance (6MWD) and the relative reduction in PVR. The proportion of patients who achieved an improvement in functional class was similar.
Questions and answers with Dr. John Swiston and Dr. John Granton
Q: Approximately, what is the percentage of inoperable vs residual PH at your clinic?
A: Dr. Swiston: Approximately 40% of patients with CTEPH in our centre are deemed inoperable. 20-30% of patients that undergo PEA have residual disease.
A: Dr. Granton: We serve as a national referral centre for CTEPH surgery. In our program 10% of patients referred for surgical consideration are felt to be inoperable. Of those that go on to surgery approximately 10-15% have evidence of residual disease. In my view all patients with evidence of chronic thromboembolism should be reviewed by an expert centre, as even minor irregularities on CT could be indicative of more widespread surgical disease.
Q: Based on these results, how would this medicine impact your care of PH patients with residual symptoms?
A: Dr. Swiston: Patients with residual symptoms would be evaluated to determine whether they have residual PH as the cause for their symptoms. If PH is present, these patients would be considered for medical therapy, including the use of riociguat.
A: Dr. Granton: Other causes for the ongoing disability should be evaluated. Additionally recurrent thromboembolism should be considered for any change in clinical condition. Recurrence in patients with unilateral disease or other thrombophilic disorders is well established. In the absence of new operable disease we would offer patients medical therapy. Based upon the CHEST-1 study, we would use riociguat for this group of patients as a first-line agent.
ABSTRACT 4060
Effect of macitentan on haemodynamics in SERAPHIN, a randomised controlled trial in pulmonary arterial hypertension (PAH)
O. Sitbon, R. Channick, M. Delcroix, N. Galiè, H. A. Ghofrani, P. Jansa, F. O. Le Brun, S. Mehta, L. Perchenet, T. Pulido, B. K. S. Sastry, R. Souza, A. Torbicki, L. Rubin, G. Simonneau (Le Kremlin Bicêtre, France; Boston, San Diego, United States Of America; Leuven, Belgium; Bologna, Italy; Giessen, Germany; Prague, Czech Republic; Allschwil, Switzerland; London, Canada; Mexico City, Mexico; Hyderabad, India; São Paulo, Brazil; ECZ-Otwock, Poland)
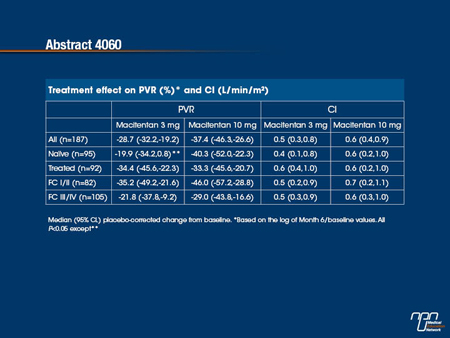
Macitentan’s effect on haemodynamics in PAH patients was assessed at selected centres in the SERAPHIN study (NCT00660179). 742 patients were randomised to placebo, macitentan 3 or 10 mg q.d. Background phosphodiesterase-5 inhibitors and oral/inhaled prostanoids were allowed. 187 patients had right heart catheterisation at randomisation and Month 6. Placebo (n=68), macitentan 3 mg (n=62) and 10mg (n=57) groups were well balanced for baseline haemodynamics with median pulmonary vascular resistance (PVR), 800, 785, 789 dyn•sec/cm5 and cardiac index (CI) 2.49, 2.23, 2.47 L/min/m2. On average, all cardiopulmonary haemodynamics improved at Month 6 with macitentan and worsened with placebo. Subgroup analyses by background PAH therapy (naïve or treated) and baseline WHO FC I/II or III/IV showed that beneficial treatment effects with macitentan remained almost entirely significant for PVR and CI.
Macitentan improved cardiopulmonary haemodynamics in PAH patients with consistent improvements in PVR and CI irrespective of background PAH therapy and baseline WHO FC.
Commentary on abstract 4060
Macitentan, an experimental dual endothelin-receptor antagonist (ERA), was recently evaluated for the treatment of pulmonary arterial hypertension (PAH) in a large Phase III trial. In this trial, called SERAPHIN, macitentan was associated with a significant benefit relative to placebo on a composite end point that include death and hospitalization as well as worsening of PAH. In the substudy analysis of that trial, the effect of this agent, relative to placebo, was evaluated on the hemodynamic parameters pulmonary vascular resistance (PVR) and cardiac index (CI). While similar in the two groups at baseline, both PVR and CI improved on macitentan but worsened in those receiving placebo at the six month evaluation. This was consistent among patients who received additional therapies for PAH and those with lower versus higher functional class. The findings strengthen the evidence that macitentan is effective for the treatment of PAH.
Questions and answers with Dr. John Swiston and Dr. John Granton
Q: How do you interpret the hemodynamic results for the different doses of macitentan?
A: Dr. Swiston: Both the 3 mg and the 10 mg doses of macitentan showed a favourable (and statistically significant) improvement in the reported hemodynamic parameters. In general, there appears to be a trend towards improved hemodynamics with increasing doses of macitentan.
A: Dr. Granton: Both doses produced a reduction in PVR and increase in cardiac output compared to placebo. However, given the potential dissociation between PVR and right heart function, it would be reassuring to see more direct evidence of improvement in cardiac function (e.g., MRI).
Q: In which patient group would you consider using macitentan?
A: Dr. Swiston: I would consider macitentan for category 1 PAH patients, WHO functional class II or greater.
A: Dr. Granton: I would also consider macitentan for category 1 PAH patient with WHO class II or greater, as initial therapy or add-on therapy.
ABSTRACT 1786
Effect of macitentan on morbidity and mortality in pulmonary arterial hypertension: A randomised controlled trial (SERAPHIN)
H. A. Ghofrani, R. Channick, M. Delcroix, N. Galiè, P. Jansa, F. O. Le Brun, S. Mehta, C. Mittelholzer, T. Pulido, B. K. S. Sastry, O. Sitbon, R. Souza, A. Torbicki, L. Rubin, G. Simonneau (Giessen, Germany; Boston, San Diego, United States Of America; Leuven, Belgium; Bologna, Italy; Prague, Czech Republic; Allschwil, Switzerland; London, Canada; Mexico City, Mexico; Hyderabad, India; Le Kremlin Bicêtre, France; São Paulo, Brazil; ECZ-Otwock, Poland)
The effect of macitentan, a novel dual endothelin receptor antagonist, on morbidity and mortality was assessed in patients with pulmonary arterial hypertension (PAH). In this double-blind, placebo-controlled, Phase III, event-driven study (SERAPHIN; NCT00660179), 742 PAH patients (≥12 years) were randomised to placebo (n=250), macitentan 3 mg (n=250) or 10 mg (n=242) once daily. 64% received PAH-specific drugs at baseline. Mean treatment duration was 85.3, 99.5 and 103.9 weeks, respectively. The primary endpoint was time from treatment initiation to first morbidity or mortality event (death, atrial septostomy, lung transplantation, initiation of i.v./s.c. prostanoids or PAH worsening – blindly and independently adjudicated). Macitentan reduced the risk of such an event vs placebo by 30% (97.5%CI: 4–48%; P=0.0108) in the 3 mg group and 45% (97.5%CI: 24–61%; P<0.0001) in the 10mg group. This effect was established early, sustained over the entire study duration and, for the 10 mg dose, was preserved across WHO functional class (FC), as well as in combination with other PAH-specific drugs. Macitentan 3 mg and 10 mg reduced the risk of PAH-related death or hospitalisation (a composite secondary endpoint) by 33% (97.5%CI: 3–54%; P=0.0146) and 50% (97.5%CI: 25–67%; P<0.0001). Macitentan was well tolerated; incidences of elevated liver aminotransferases and peripheral oedema were similar across groups. Headache, nasopharyngitis and anaemia occurred more frequently with macitentan than placebo. In conclusion, macitentan significantly reduced morbidity and mortality in patients with PAH, with a favourable safety profile.
Commentary on abstract 1786
The SERAPHIN trial, which compared the experimental endothelin-receptor antagonist (ERA) macitentan to placebo in patients with pulmonary arterial hypertension (PAH), was the first Phase III trial in PAH to evaluate efficacy on the basis of a reduction in clinical events rather than improvement in exercise capacity. The composite event end point included PAH-related mortality and hospitalization as well as objective evidence of worsening of PAH. In this analysis, a more detailed breakdown of specific morbidity and mortality was undertaken, comparing both the 10 mg and 3 mg study doses of macitentan to placebo. There was good tolerability for both doses, but outcomes were better on the 10 mg dose. Headache, nasopharnyngitis, and anemia, were the most common adverse events. On the prespecified combined secondary end point of PAH-related mortality or hospitalization, the risk reductions relative to placebo were 50% for the 10 mg dose (P<0.0001) and 33% for the 3-mg dose (P=0.0146). The evidence of efficacy and safety in this analysis supports regulatory approval.
Questions and answers with Dr. John Swiston and Dr. John Granton
Q: What is your interpretation of the morbidity and mortality composite end point and how does this affect your clinical practice?
A: Dr. Swiston: The strength of a composite end point is that it encompasses a number of outcomes or clinical interests, its weakness lies in the fact that it is only as convincing as the weakest (and/or most frequent) clinical parameter. In the case of the SERAPHIN trial, the most frequent primary end point event was worsening pulmonary hypertension. However, this end point was well designed for clinical relevance and subjected to blind adjudication in the SERAPHIN trial and therefore provides compelling evidence that therapy with macitentan can produce a significant positive impact on PAH.
A: Dr. Granton: Composite end points help build the case for benefit in a trial evaluating treatments of uncommon conditions, where mortality is either rare or a direct mortality benefit is difficult to prove, within the confines of the study. As mentioned, the results of any study using a composite score are usually driven by one of the components of the composite end point. Therefore, each of the components should be clinically relevant and have similar directionality. In the SERAPHIN study, the components of the composite score were the result of a thoughtful scientific review and regulatory input. It is reassuring that the outcomes were blindly adjudicated. The most frequent primary end point was worsening of PAH. In order to be classified as "worsening of PAH" the patient had to have: 1) a decrease in the 6-minute walk distance of at least 15% from baseline, 2) a worsening of symptoms of PAH (which included at least one of worsening in WHO functional class or persistent class IV, and refractory signs of right-sided heart failure), and 3) the need for additional treatment for PAH. A composite end point of death due to PAH or hospitalization for PAH also demonstrated a treatment benefit that appeared to be dose dependent. Two sensitivity analyses, performed to deal with deaths or missing data, confirmed the effect on the primary composite end point. Overall the results support the notion that this agent is effective in the treatment of PAH, by evaluating effect on relevant outcomes over a longer period of time, as compared to historical clinical trials in PAH.
Q: In what way do you think this trial should impact future trial design?
A: Dr. Swiston: I think that this trial design will have a significant impact on future clinical trials in PAH by demonstrating that it is feasible to perform studies with greater clinical relevance in this patient population.
A: Dr. Granton: This trial design aligns with recommendations from several PAH scientific bodies, specifically that the effect of novel medications on patient relevant outcomes, over a longer period of observation, need to be completed. This trial will likely become the benchmark for future trials in PAH. However, with increased interest in right ventricular function, future trials should consider inclusion of a more direct evaluation of cardiac function, e.g., MRI. In the future, it will also be desirable to provide some reassurance that medications are truly disease-modifying.
ABSTRACT 2627
Pulmonary vascular reactivity in pulmonary hypertension due to left heart disease
C. Gerges, M. Gerges, M. Lang, J. Mascherbauer, I. Lang (Vienna, Austria)
Purpose: Pulmonary hypertension (PH) due to left heart disease (LHD) is the most common subset of PH. It is defined by an increase of mean pulmonary artery pressure (mPAP) ≥25mmHg in the presence of a mean pulmonary capillary wedge pressure (mPCWP) >15mmHg. In the current guidelines PH due to LHD with a TPG >12mmHg is labeled as "out-of-proportion" PH, as opposed to what is labeled as "passive" PH. Recent data have shown that patients with "out-of-proportion" PH and a diastolic pulmonary vascular pressure gradient (DPG) ≥7mmHg have an increased mortality and significant pulmonary vascular disease. We hypothesize that these patients may benefit from vasodilator treatment. The aim of this study was to compare the degree of acute vasoreactivity to inhaled nitric oxide (NO) in "out-of-proportion" PH with a DPG ≥7mmHg to that of "passive" PH and "out-of-proportion" PH with a DPG <7mmHg.
Methods: A prospective data set of 94 patients with PH due to LHD undergoing first diagnostic right heart catheterizations at rest and after inhalation of 40ppm NO was analyzed. 31 patients were classified as "passive" PH, 28 as "out-of-proportion" PH with a DPG <7mmHg and 35 as "out-of-proportion" PH with a DPG ≥7mmHg.
Results: The strongest decrease of mPAP was observed in patients with "out-of-proportion" PH and a DPG ≥7mmHg (-5.1±4.7mmHg, p<0.001). In contrast there was no significant change in mPAP in patients with "out-of-proportion" PH and a DPG <7mmHg (-2.2±5.9mmHg, p=0.075). In "passive" PH mPAP did not change upon NO inhalation.
Conclusion: DPG identifies patients with "out-of-proportion" PH who have significant pulmonary vascular disease that is reactive to inhaled NO.
Commentary on abstract 2627
Left heart disease with pulmonary hypertension (PH) is defined as a mean pulmonary artery pressure (mPAP) of >25 mmHg in the presence of a mean pulmonary capillary wedge pressure of >15 mmHg. When the transpulmonary gradient (TPG) is >12 mmHg, it is considered to be out-of-proportion PH. Responding to recent evidence that a diastolic pulmonary vascular gradient (DPG) may provide additional information in patients with out-of-proportion PH, the degree of acute vasoreactivity to inhaled nitric oxide (NO) in 35 patients with a DPG ≥7mmHg was compared to the reactivity in 28 patients with a DPG <7mmHg. Based on the relatively larger decrease in mean pulmonary arterial pressure (mPAP) on NO in those with the higher DPG, this may be a useful marker of those with significant pulmonary vascular disease that is reactive to NO.
Questions and answers with Dr. John Swiston and Dr. John Granton
Q: In patients with left heart disease, are there key hemodynamic characteristics that are useful for differentiating this group from those with right heart disease?
A: Dr. Swiston: Any hemodynamic characteristics must be interpreted in a clinical context. That being said, an important hemodynamic parameter to identify patients with significant left heart disease is the pulmonary capillary wedge pressure (PCWP). There are a number of recent studies that suggest that DPG will also be a valuable tool to discriminate between patients with category 1 and 2 PH, and explore those that may have a combination of the two.
A: Dr. Granton: Efforts to better define these subgroups of patients are to be commended. They are a necessary step to identify patients and systematically evaluate current treatments in a controlled fashion. In addition to DPG, response to exercise or fluid challenges may also help in distinguishing patients with impaired diastolic function.
Q: Does an increased diastolic pressure gradient, at the level described in this study, outline a subgroup of patients with left heart PH who may be responsive to vasodilators despite previous reports of disappointing results with vasoreactive therapies?
A: Dr. Swiston: While the hypothesis and results of this study are interesting, it is a very small exploratory study. Previous studies have suggested that a DPG cutoff of 7 delineates patients that may have different physiology. Whether the hemodynamic findings of this study are reproducible in larger studies and have clinical relevance remains to be seen.
A: Dr. Granton: This really needs to be addressed in properly conducted trials with assurances that the patients being evaluated are well defined. At present, the benefits of pulmonary vasodilators on outcomes that are relevant to patients in this category is uncertain.
ABSTRACT 2602
Symptom-related telephone monitoring and cardiopulmonary exercise testing in the pulmonary embolism follow-up for early detection of chronic thromboembolic pulmonary hypertension (CTEPH)
M. Held, A. Hesse, R. Holl, T. Romen, F. Walter, G. Huebner, B. Jany (Wuerzburg, Germany)
The incidence of CTEPH as a serious complication of pulmonary embolism (PE) varies between 0.5 -8%. There are currently no established structured follow-up programs for early detection of CTEPH.
We prospectively studied the follow-up of patients with newly diagnosed PE to evaluate a symptom-related approach which is based on a telephone monitoring program. Patients were contacted after three, six, 12, 24 and 36 months. Patients were further studied if one item of a five item-questionnaire was positive. further imaging studies and right heart catheterization were performed in case that echocardiography or/and cardiopulmonary exercise testing (CPET) revealed abnormalities suggestive of pulmonary hypertension.
We report the results from our 3 months- follow-up and 18- months interim analysis.
3-months follow-up: n=104. Telephone interview suggesting abnormalities: n=32 (29,8 %). Pathological echocardiography: n=7, normal n= 25: CPET n=20: pathological CPET n =7, normal n =13. Further diagnostic work-up: n=15. Diagnosis CTEPH: n=7.
Interim analysis after18 months: n =123:Diagnosis CTEPH: n=10 .3 out of 10 patients had shown a pathological CPET despite normal echocardiographic findings.
The symptom-related follow-up program which is based on a telephone-monitoring and a 5 item-questionnaire detects patients with chronic-thromboembolic pulmonary hypertension. Cardiopulmonary exercise testing may serve as a complementary diagnostic tool. Telephone monitoring and cardiopulmonary exercise testing should be included in a pulmonary embolism follow-up program for early detection of CTEPH.
Commentary on abstract 2602
Chronic thromboembolic pulmonary hypertension (CTEPH) is an uncommon but serious complication of pulmonary embolism (PE). Early diagnosis may improve opportunities for successful pulmonary endarterectomy or other interventions that can prevent progression leading to permanent lung damage. When symptoms are mild, CTEPH can go undetected for prolonged periods, limiting opportunities for successful intervention. This study evaluated a telephone monitoring system in which patients with a prior PE were asked about symptoms of CTEPH using a 5-item questionnaire. A single positive response was sufficient to prompt an office visit for further study. After PE was evaluated. Patients were contacted at 3, 6, 12, 24, and 36 months after their PE. In this interim analysis of 123 patients evaluated over 18 months, 10 CTEPH patients were identified. In three cases, the CTEPH was detected on cardiopulmonary exercise testing when echocardiographic studies had been negative. The results of the study support telephone-based monitoring for increasing CTEPH detection rates after PE.
Questions and answers with Dr. John Swiston and Dr. John Granton
Q: Is there a need for this type of program in Canada?
A: Dr. Swiston: There is a need to recognize patients at risk for CTEPH and to initiate appropriate investigations if these patients do not fully recover or develop exertional limitation following an episode of acute pulmonary embolism.
A: Dr. Granton: Strategies to properly identify patients at risk for or who have CTEPH are needed. This condition is likely underappreciated and as a result patients may be losing the opportunity to benefit from surgical intervention – a potentially curative procedure.
Q: What can we do to improve diagnosis in CTEPH patients?
A: Dr. Swiston: Improving the diagnosis of CTEPH requires increased awareness of this disease as a complication of acute pulmonary embolism. The diagnosis should be considered in any patient with otherwise unexplained dyspnea and a history of PE.
A: Dr. Granton: I agree. There is a need to educate our colleagues and our patients about the signs of chronic disease. Studies, such as the recently completed ELOPE (a CIHR-sponsored Canadian trial,
led by Dr. Susan Khan), evaluating the consequence of pulmonary embolism on exercise capacity and quality of life, as well as registries, are needed to provide us with a better understanding about the impact of PE on patients. At present it is unclear what type (and intensity) of follow-up is required for patients with newly diagnosed PE. However, it may well be that recognition of persistent symptoms following PE warrant further evaluation.
ABSTRACT 4081
Right ventricular dysfunction in pulmonary hypertension with combined pulmonary fibrosis and emphysema syndrome
A. Swift, S. Rajaram, D. Capener, C. Hill, C. Davies, J. Hurdman, R. Condliffe, C. Elliot, D. Kiely, J. Wild (Sheffield, United Kingdom)
Introduction: Recent studies suggest that the coexistence of emphysema and fibrosis alters clinical outcome. The aim of this study was to investigate the comparative clinical characteristics, pulmonary function, haemodynamics and right ventricular (RV) function and outcome in patients with pulmonary hypertension associated with combined pulmonary fibrosis and emphysema (PH-CPFE), chronic obstructive pulmonary disease (PH-COPD) and interstitial lung disease (PH-ILD).
Methods: In 79, incident patients with pulmonary hypertension associated with respiratory disease, cardiovascular magnetic resonance imaging was performed at 1.5T. Emphysema and fibrosis were qualitatively assessed on high resolution computed tomography scans as previously described. Pulmonary function tests and right heart catheterisation were also performed.
Results: Patients with pulmonary hypertension associated with combined pulmonary fibrosis and emphysema syndrome had a significantly lower right ventricular ejection fraction (RVEF) and a larger right ventricular end-systolic volume index (RVESVI), when compared to both patients with PH-COPD and PH –ILD (p<0.05). A weak, yet statistically significant association between right ventricular dysfunction and the combined emphysema fibrosis score was identified (RVEF; r= -0.25, p=0.038). Whereas, pulmonary function test data were not significantly associated with RV dysfunction. At Kaplan Meier analysis, patients with PH-CPFE patients had worse outcome than those with PH-COPD (p=0.024) and borderline worse outcome than PH-ILD (p=0.067).
Conclusions: Patients with PH-CPFE have poor outcome with worse RV function in comparison to patients with PH-COPD and PH-ILD.
Commentary on abstract 4081
Patients with either COPD or ILD are known to be at risk for the development of PH. The characteristics and natural history of PH in the subgroup of patients with both COPD and ILD has not been well described. In this evaluation, hemodynamics and cardiac function in such patients were compared to patients with PH and either chronic obstructive pulmonary disease (PH-COPD) or or PH with interstitial lung disease (PH-ILD) alone. The study of 79 patients found that those with fibrosis and emphysema had worse right heart function and poorer outcome than either of the other two groups. Although the difference in outcome fell just short of significance relative to PH-ILD (P=0.067), the difference did reach significance for PH-COPD (P=0.024). This study suggests that identifying the presence of both fibrosis and emphysema in patients with PH may yield prognostic information and highlights the need to further study this subpopulation of patients.
Questions and answers with Dr. John Swiston and Dr. John Granton
Q: Based on evidence of worse outcome in patients with PH-CPFE, do you see any opportunities for more aggressive care in this population?
A: Dr. Swiston: There are currently no approved therapies for PH associated with either ILD or COPD and certainly none with CPFE. These patient populations should receive optimal care for both their ILD and/or COPD. PH associated with these two diseases alone or in combination should be the focus of further analysis.
A: Dr. Granton: This is a challenging group of patients to manage as they have a worse prognosis, are often more hypoxemic, having advanced symptoms and disability. Early referral for consideration of lung transplant should occur in suitable patients. At present the role of specific treatment for pulmonary vascular complications (apart from long-term oxygen therapy) is unclear.