Reports
Remission with Complete Mucosal Healing Is the Emerging Treatment Goal for Ulcerative Colitis and Crohn’s Disease
This report is based on medical evidence presented at sanctioned medical congress, from peer reviewed literature or opinion provided by a qualified healthcare practitioner. The consumption of the information contained within this report is intended for qualified Canadian healthcare practitioners only.
MEDICAL FRONTIERS - 2011 Advances in Inflammatory Bowel Diseases Crohn’s and Colitis Foundation (CCF) Clinical & Research Conference
Hollywood, Florida / December 1-3, 2011
Hollywood - In patients with an inflammatory bowel disease (IBD), whether ulcerative colitis or Crohn’s disease, outcome is superior when remission includes mucosal healing. The data are so persuasive that there is now an exploration of the concept of remission with complete mucosal healing, which applies to normal or near normal histologic recovery. The principle of mucosal healing is that greater
degrees of disease quiescence provide a greater barrier to relapse. Relative to partial resolution of inflammatory activity in IBD, which appears to be more readily triggered into periodic clinical flares, profound levels of disease control appear to render less likely both the initiation of the cascade of inflammatory signals and a resurgence of disease activity. IBD therapies were once guided by symptom
control, but symptom relief and healing have a limited correlation. Healing is therefore an important goal for a chronic disease in which long-term outcomes must be considered in any treatment scheme.
Chief Medical Editor: Dr. Léna Coïc, Montréal, Quebec
The premise of mucosal healing in inflammatory bowel disease (IBD), which is now being considered by the U.S. Food and Drug Administration as a therapeutic target when demonstrating efficacy in IBD, is that risk of relapse declines in parallel with resolution of inflammatory activity. Currently, the best evidence of inflammatory control is mucosal healing, although other markers, including serum
assays of circulating inflammatory mediators or a return to normal or near normal histology, may be useful. The common goal in all strategies is achieving a degree of disease quiescence that will provide a higher barrier to relapse.
“Over the long term, we need to ask ourselves how we are going to keep patients from progressing to hospitalization and colectomy,” suggested Dr. David T. Rubin, University of Chicago, Illinois. “One way will be to substitute objective goals for symptombased control. Another is to focus on long-term outcomes rather than short-term management.”
Corroborative Evidence
Speaking specifically about the control of ulcerative colitis (UC), Dr. Rubin emphasized that the treatment goal for an acute flare should now be mucosal healing. This is based on evidence that less rigorous end points increase the risk for relapse and serious adverse complications from progressive disease, such as hospitalization and surgery. Importantly, symptom control may be achieved without
healing, but healing may also take place without complete symptom control. In the phase III ACT studies with infliximab in UC, healing at 8 weeks was twice as great on the TNF inhibitor relative to placebo (62% vs. 34%; P<0.001) but within the infliximab group, the proportion healed was 50% greater than those with complete symptom relief (62% vs. 39%; P<0.001) (Figure 1). This makes symptom control a particularly imprecise measure of treatment success.
Figure 1.
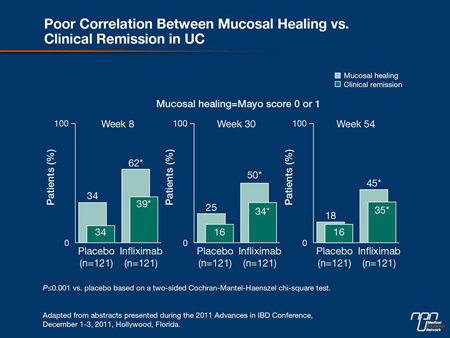
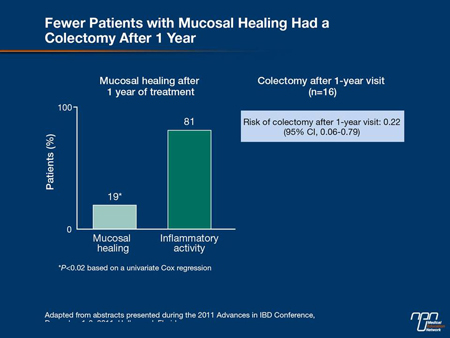
“We now have a great deal of data to tell us that healing on endoscopy is our treatment goal in the acute management of UC. The trials have demonstrated the benefits of mucosal and histologic healing in UC with reductions in hospitalization, reductions in surgery and reductions in neoplasia, so ultimately, that is indeed our goal,” agreed Dr. Stephen B. Hanauer, University of Chicago. He cited a study in which the relapse rate at 1 year was 80% in those with a clinical remission defined by symptoms but 23% (P<0.0001) in those with a clinical and endoscopic remission. In another, the rates of colectomy during lifetime followup were 19% vs. 81% (P<0.02) in those who did or did not have mucosal healing 1 year after initial therapy (Figure 2, 3).
Figure 2.
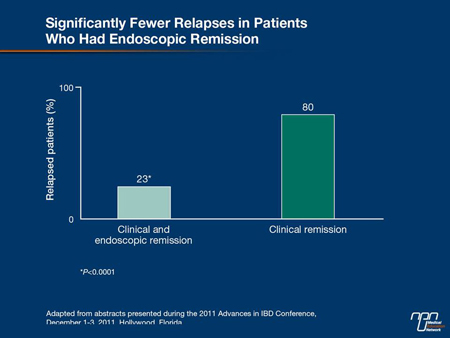
Figure 3.
Mucosal Healing, Lower Relapse Risk
The improved outcomes in patients who achieve healing are not specific to treatment, even though the likelihood of healing in patients with severe disease is clearly related to use of the most effective therapies. In mild to moderate UC, mucosal healing (Figure 4)—
which is achieved in up to 32% of patients at 8 weeks with controlled-release products—predicts a lower relapse at 1 year than partial healing. In patients not healed on first-line therapy, Dr. Hanauer urged that patients be rapidly stepped up to the other options which include azathioprine (AZA), 6-mercaptopurine (6-MP), corticosteroids or TNF inhibitors. According to Dr. Hanauer, the same relationship between healing and improved outcome is observed with each UC treatment. He cited a study with corticosteroids in which 49% of those with complete healing were relapse-free at 1 year. In contrast, prolonged responses in those with a partial remission were uncommon and 22% were steroid-dependent after 1 year. In those without a response to corticosteroids at 1 month, 29% had gone on to surgery by 1 year (Figure 5).
Figure 4.
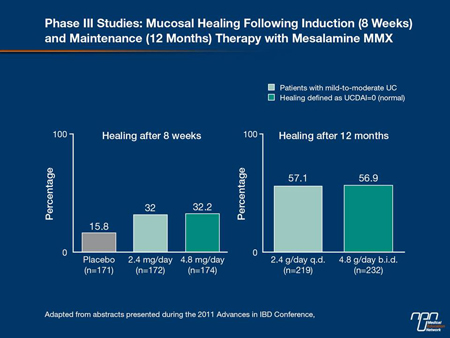
Figure 5.
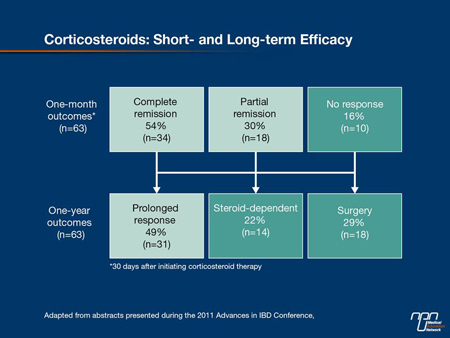
Figure 6.
“The appreciation for the implications of a healed mucosa has contributed to an evolving approach to UC that includes early adoption of sequential therapies, including biologics, to reach this goal,” Dr. Hanauer told delegates.
Findings from SUCCESS
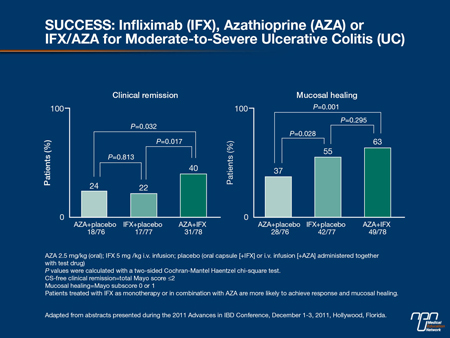
In patients with moderate to severe UC, mesalamine is not an appropriate first-line therapy. The question of whether to start with an immunosuppressant such as AZA, a biologic or both was addressed in an important study called UC SUCCESS, which was presented at the 2011 European Crohn’s and Colitis Organization (ECCO) meeting (Panaccione et al. J Crohn’s Colitis 2011;5:S80, Abstract 13). The 16-week, double-blind trial recruited 231 biologic-naïve patients with a Mayo score of at least 6. For eligibility, patients had to be failing corticosteroids and either naïve to AZA or had stopped AZA at least 3 months before entry. Subjects were randomized to AZA 2.5 mg/kg plus placebo, infliximab 5 mg/kg plus placebo or to infliximab 5 mg/kg plus AZA 2.5 mg/kg. Nonresponders (Mayo score reduction <1 point) on the AZA arm at week 8 were eligible to receive infliximab subsequently.
The healing rates at 16 weeks climbed steeply in those receiving infliximab relative to those receiving AZA (55% vs. 37%; P=0.028). There was a further climb in those receiving both active therapies relative to infliximab alone (63% vs. 55%; P=0.295) (Figure 6). Although this difference did not reach statistical significance, Dr. Hanauer indicated that the stepwise improvement would support the advantage of moving quickly to more aggressive therapy in patients with moderate to severe UC.
Indeed, there is some evidence that the speed at which inflammation is suppressed is also a prognostic indicator. In a placebo-controlled study with adalimumab, 71% of UC patients on the TNF inhibitor vs. 38% of those in the placebo group that had healing at 8 weeks were in remission at 1 year (Figure 7). Although this did not have another active treatment arm to prove that relative ability to achieve faster healing predicts increased longterm remission, it is consistent with the concept that the ability to efficiently turn off inflammatory activity is an important prognostic indicator.
“The data from this study and others suggests that therapies able to produce mucosal healing in the short term have a high likelihood of preserving remission in the long term,” reported Dr. Edward V. Loftus, Mayo Clinic, Rochester, Minnesota. These data suggest that
it is important to recognize aggressive disease and treat it with a strategy appropriate to the likelihood of achieving and then maintaining mucosal healing.
Figure 7.

Adverse prognostic factors may include refractoriness to previous therapies, deep endoscopic lesions as well as biomarkers, such as low albumin or elevated C-reactive protein (CRP).
Eventually, this may include individualizing therapy according to biomarkers of severity. Although there is not yet good evidence that biomarkers may guide therapy in UC, Dr. Hanauer cited trial data from a Crohn’s disease (CD) study that found CRP levels to distinguish likelihood of benefit from more frequent dosing (Figure 8).
Figure 8.
ACT Studies
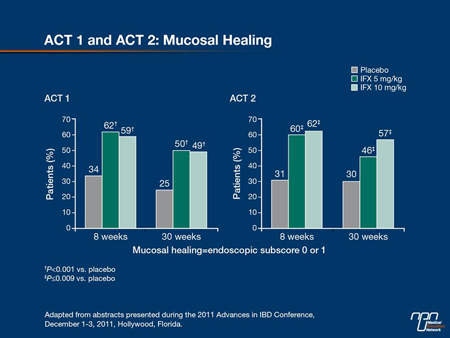
In UC, the ACT trials have been perhaps the most influential in encouraging earlier use of the most effective therapies. In these trials published 6 years ago (Rutgeerts et al N Engl J Med 2005;353:2462-76), patients with moderate-to-severe active UC despite treatment with concurrent medications were randomized to placebo, infliximab 5 mg/kg or infliximab 10 mg/kg administered at weeks 0, 2 and 6 and then every 8 weeks. After 46 weeks, patients in ACT 1 were followed for a year. After 54 weeks, patients in ACT 2 were followed
for 30 weeks. In both studies, mucosal healing was achieved at week 8 in approximately 60% of patients randomized to either dose of infliximab (Figure 8). This was approximately double the rate of healing in the placebo group and the highest rate of healing ever
observed in a UC study.
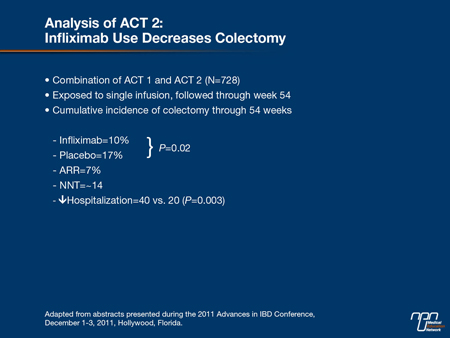
“Perhaps the most important result was the difference in cumulative colectomy rates at the end of 54 weeks, which were almost twice as high in the placebo group [17% vs. 10%; P=0.02],” Dr. Hanauer reported (Table 1). He indicated that this study has been instrumental in the evolution of treatment goals in UC management, particularly pursuing strategies that will reduce long-term complications in a disease that has frequent recurrences and a propensity toward progression.
Table 1.
The lack of correlation between healing and symptom relief in the ACT trials underscores the reason that objective measures of treatment response are needed. While some studies with mesalamine in patients with mild to moderate disease suggest
adequate symptom relief in the absence of complete healing, the greater rate of healing relative to symptom relief with the TNF inhibitors still predicts a lower risk of complications. Controlling symptoms is important for quality of life, but healing is important for reducing
risk of subsequent surgery.
“About 20% of patients have irritable bowel symptoms on top of their IBD symptoms. As a result, many patients with CD and UC have residual symptoms despite endoscopic healing, but these symptoms may not be based on inflammation,” Dr. Hanauer explained. In the context of improved outcomes with healing, this is an important concept because it establishes endoscopic healing as a goal independent of other measures of treatment efficacy.
Step Up Therapy
In UC, there has been a relative reluctance to step up therapy at the same pace in refractory patients as there has been in CD. However, the nature of the inflammatory cascade is similar, and the principles of management in regard to suppressing the underlying mediators of disease to improve long-term outcome are the same. A substantial proportion of patients with mild to moderate UC will respond to mesalamine, which is well tolerated and has been associated with a reduced risk of colon cancer in epidemiologic studies. Corticosteroids are effective in achieving symptom control, yet several investigators, including Dr. William J. Sandborn, University of California, San Diego, have suggested that these may be best used as induction agents because of relatively low rates of healing in controlled trials.
“There has been increasing interest in controlledrelease budesonide which appears to have fewer serious toxicities than conventional steroids. This drug is modestly effective as a maintenance therapy in UC, but it has shown to be effective as an induction agent in regard to clinical remissions,” Dr. Sandborn told delegates. He indicated that it might be used to step up response rates with mesalamine or an immunotherapy before considering a biologic.
Screening for C. difficile
Regardless of treatment, one fundamental step to adequate management of UC is screening for and treatment of Clostridium difficile. Dr. Sandborn noted that although UC-related mortality is uncommon, it does occur in association with C. difficile infections which can exacerbate disease and prevent healing with otherwise effective therapies. Collating data from 3 different studies conducted between 1997 and 2007, he said the in-hospital mortality in patients with C. difficile is increased by about fourfold. The rates of colectomy have also been increased although not significantly.
“Even in a patient who has been previously tested for C. difficile, I may consider screening again if there is change in their response to maintenance therapy,” Dr. Sandborn confirmed.
Patient, Physician Expectations of Healing
It should now be well accepted that objective treatment goals, particularly healing, should be substituted for the symptom relief once used to judge treatment efficacy in UC. It is equally important to recognize that there is also variability in disease course. Both patients
and physicians need to recognize that tighter control of the inflammatory cascade is important for longterm outcomes. Citing a survey in 451 UC patients, Dr. Rubin reported that 80% of patients believe flareups are normal, and he believes that many patients who report adequate control at office visits do not necessarily report flare-ups that occur between visits and may represent diminishing disease control.
“There is an important disconnect between health care providers and patients in regard to expectations, and we, as physicians, may not always be soliciting enough information about ongoing disease activity,” Dr. Rubin warned. Due to the importance of downregulating inflammatory activity, he indicated that tighter standards of treatment success have implications for long-term outcome.
Summary
In UC as in CD, the criteria for effective treatment of acute flares are becoming more stringent because of the evidence that control of inflammatory activity predicts a lower long-term risk of complications, including hospitalization and colectomy. The importance of
mucosal healing for predicting outcome appears to be independent of the treatment used. This is consistent with the concept that the inflammatory cascade is less likely to reignite at greater degrees of relative quiescence. This concept is fundamental to the algorithms required to prevent long-term complications.