Reports
The Microbiome and the Brain-Gut Axis: A Role for Prebiotics and Probiotics
This report is based on medical evidence presented at sanctioned medical congress, from peer reviewed literature or opinion provided by a qualified healthcare practitioner. The consumption of the information contained within this report is intended for qualified Canadian healthcare practitioners only.
PHYSICIAN PERSPECTIVE - Viewpoint based on presentations and expert opinion from the New York Academy of Sciences (NYAS) Probiotics, Prebiotics, and the Host Microbiome: The Science of Translation and the 11th Annual Meeting of the International Scientific Association for Probiotics and Prebiotics (ISAPP)
New York, New York / June 12-14, 2013
Reviewed and Edited by:
Eamonn Quigley, MD
Chief, Division of Gastroenterology and Hepatology
The Methodist Hospital and Weill Cornell College of Medicine
Houston, Texas
Introduction
The influence of gut microbiota on hormone and neurotransmitter signaling, functions that may be modulated by the central nervous system (CNS), is now understood to offer major opportunities to improve human health. The importance of the brain-gut axis on digestion, metabolic processes, and visceral pain sensation has long been recognized, but the evidence that prebiotics and probiotics can favorably influence these interactions is an exciting focus of new treatment development. Two related conferences, a symposium on prebiotics and probiotics presented by the New York Academy of Sciences (NYAS) followed immediately by the invitation-only 11th Annual Meeting of International Scientific Association for Probiotics and Prebiotics (ISAPP), provided a basis to consider the clinical direction of this research. Data summarized at these meetings documented a shift from attention on prebiotics and probiotics on localized effects in the bowel to the ability of these agents to favorably influence disrupted brain-gut signaling fundamental to a broad range of pathologies.
The term gut microbiome, which refers to the totality of the interaction of microbe and human genomes in the gastrointestinal (GI) tract, recognizes that normal as well as abnormal physiologic function is dependent on microbe-host relationships. While a symbiotic relationship between resident gut flora, nutrient absorption and other aspects of digestion has long been recognized,1 it is now clear that gut flora mediates a broad host of functions, including homeostasis of the immune system and neurohormone signaling along the brain-gut axis.2 Gut flora are now even suspected of a role in the etiology of such diverse pathological states as obesity and cardiovascular disease.3-4 Recent progress, accelerated by advances in the ability to characterize proteomics of microbes as they relate to both gut and systemic function, suggest new opportunities to intervene with prebiotics and probiotics in a broad range of pathophysiologic processes.
The microbial counts in the GI tract, which is colonized at infancy,5 vary by segment, reaching as high as 1012 cells/g of luminal contents in the colon.6 Microbial cells in the gut microbiome outnumber human cells by a ratio of 10 to 1, suggesting the microbial genome is likely to be more important than the human genome in creating or disturbing homeostasis in this environment.7 The influence of the gut microbiome on human physiology is now thought to be so broad and diverse that it has been suggested that this entity is best addressed as an independent organ.8
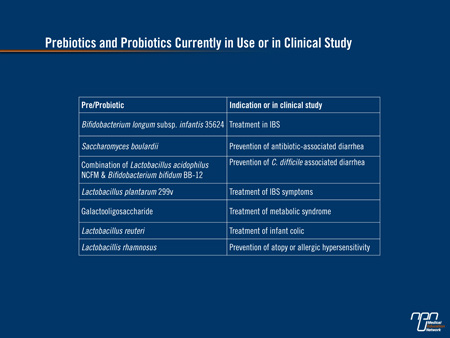
Prebiotics and probiotics are considered by many to exist at some intermediate step between drugs and food. Prebiotics, first described in 1995,9 are non-digestible organic products that stimulate growth of bacteria in the digestive system usually by providing nourishment. Probiotics are live bacteria or yeasts introduced into the digestive tract for an anticipated benefit. Examples with proven activity include infantis subspecies of Bifidobacterium longum (B. infantis) for the treatment of irritable bowel syndrome (IBS) and Saccharomyces boulardii for the treatment of acute diarrhea.10-11 These and other examples of prebiotics and probiotics currently in use or under investigation can be seen in (Table 1).
Table 1.
Microbiome and Brain-Gut Axis
The ability of the CNS to communicate with the gut can be assumed empirically from a close relationship between emotional state and gut function.12 The clinical relevance of this communication has been demonstrated by the increase risk of GI diseases and conditions, such as IBS,13 during periods of increased stress. It is, however, the increasing appreciation for bidirectional communication that is driving efforts to use prebiotics and probiotics to control disease.
Positive results with probiotics in IBS is a good illustration of the potential complexity of the bidirectional brain-gut axis. In a multicenter randomized double-blind trial that led to a commercial product for IBS, the probiotic B. infantis 35624 in encapsulated form, was significantly superior (P<0.02) to placebo for the primary efficacy variable of abdominal pain as well as a composite score for bloating, bowel dysfunction, incomplete evacuation, straining, and passage of gas.14 The side effect profile, typical of probiotics, was no different from placebo. Although the ability of the probiotic to exert a localized influence on motility is one explanation of benefit, the potential effect of a change in microbiota on CNS-mediated neuromuscular activity and sensory perception introduces others. More complex theories incorporate changes in microbiota with favorable changes on immune modulation and gut function.15
Emerging data identifying a relationship between gut microbiota and CNS-mediated metabolism are equally interesting and potentially more profound. Unlike its impact on GI physiology, the brain-gut axis at this level may exert a fundamental influence on life-long risk of systemic diseases. Published data and updates presented here, have associated disturbances in microbiome assembly through early childhood exposure (<6 months of age) to antibiotics with increased risk of subsequent weight gain.16 Animal studies support this risk and have identified specific shifts in the microbiome that are implicated in risk of weight gain.17
If further data corroborate that a shift in microbiome from antibiotic exposure in infancy promotes weight gain by one or more mechanisms, such as altering hormonal signaling for appetite, it would likely lead to a reconsideration of infection management in young children. This relationship is not confined to children and there is also data to suggest that a shift in the microbiome may also lead to obesity in adults.18 In one recent study, an antibiotic-related shift in the GI microbiota was associated with a change in enzymatic metabolism of dietary sugars consistent with impairments observed in obese individuals.19
Obesity by itself is an etiologic factor in a broad number of pathologic states affecting the circulatory, cardiovascular and renal systems, but there is also evidence that gut microbiota alters signaling fundamental to metabolic endotoxemia, a potential etiology for many chronic diseases including both diabetes and obesity.20 This research has led to isolation of Akkermansia muciniphila as a potential target of prebiotics or probiotic therapy due to evidence that it induces intestinal epithelium to improve its barrier function, thereby reversing endotoxemia.21 While these experiments remain restricted to the experimental setting, they contribute confirmatory data that gut microbiota do participate directly and fundamentally in metabolic processes.
Clinical Initiatives in Prebiotics and Probiotics: Current Examples
Despite the relatively short time that attention has been directed to targeting gut microbiota to treat human diseases, the diversity of clinical initiatives in this area is remarkable. Until relatively recently, clinical trials were largely restricted to diseases involving the GI tract, particularly diarrhea or diseases associated with impaired bowel function such as IBS and inflammatory bowel disease (IBD). While work in GI diseases is continuing, the scope of the clinical applications is being greatly expanded, based on data presented here.
In these abstracts, preliminary clinical data was presented on the effect of daily probiotics on the frequency and intensity of migraine attacks, renal function in patients with chronic kidney disease, milk metabolism in otherwise lactose intolerant individuals, delivery of vaccine antigens in remote settings where access to vaccines is challenging, and down-regulation of inflammatory markers in aging individuals. In an ambitious study called PAMS, individuals at risk of developing metabolic syndrome were randomized to a prebiotic or placebo. Laboratory markers of progression, such as lipids, inflammatory cytokines, and lipopolysaccharide levels were monitored.
In preclinical studies presented in abstract form, the list of conditions for which a theoretical benefit has led to the development of a prebiotics or probiotic treatment strategy is now being tested in animal or culture models. Conditions include menopause-related osteoporosis, dental cavities, hyperlipidemia, and heart failure.
All of these efforts are being conducted while fundamental but not necessarily disease-specific questions are being addressed. In particular, the absence of a definition of a healthy gut microbiome is driving research initiatives to determine whether such a definition is possible. There are also multiple strategies being pursued to quantify microbiota populations. Such tools may be critical in the diagnosis of aberrant patterns and whether patients are candidates for specific prebiotics or probiotic therapies. This is likely to be a complex undertaking. Multiple factors, such as the ratio of microbes within the microbiome, may have an influence on risk in ways not necessarily measured with simple microbiota probes.
There are complex regulatory issues involving the development of prebiotics and probiotics that may also require resolution as this field moves forward. The extent to which these agents are considered drugs rather than food or dietary supplements will exert a large impact on the speed and process of clinical testing and development. While a drug designation that demands phase III testing and Food and Drug Administration (FDA) approval is likely to slow development, this limitation must be weighed against the advantage of trials that yield level one evidence of safety and efficacy.
Conclusion
The evidence that the gut microbiome is a major mediator of human physiologic function and risk of pathological states has the potential to become one of the most exciting developments in the history of medicine. This is an area of intense scientific discovery likely to develop along multiple parallel tracks as the full implications are explored. Understanding the brain-gut axis is fundamental to much of this research. While the benefit of prebiotic and probiotic therapy and the bidirectional communication along this axis has already established relevance in GI function, such as in the treatment of IBS, its independent importance to hormonal signaling that leads to metabolic diseases may prove to be the key to better understanding many of the greatest threats to public health.
References
1. Guarner F, Malagelada JR. Gut flora in health and disease. Lancet 2003;361(9356):512-9.
2. Rhee SH, Pothoulakis C, Mayer EA. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat Rev Gastroenterol Hepatol 2009;6(5):306-14.
3. Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006;444(7122):1027-31.
4. Holmes E, Loo RL, Stamler J, et al. Human metabolic phenotype diversity and its association with diet and blood pressure. Nature 2008;453(7193):396-400.
5. Harmsen HJ, Wildeboer-Veloo AC, Raangs GC, et al. Analysis of intestinal flora development in breast-fed and formula-fed infants by using molecular identification and detection methods. J Pediatr Gastroenterol Nutr 2000;30(1):61-7.
6. Simon GL, Gorbach SL. Intestinal flora in health and disease. Gastroenterology 1984;86(1):174-93.
7. Bengmark S. Ecological control of the gastrointestinal tract. The role of probiotic flora. Gut 1998;42(1):2-7.
8. Eckburg PB, Bik EM, Bernstein CN, et al. Diversity of the human intestinal microbial flora. Science 2005;308(5728):1635-8.
9. Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr 1995;125(6):1401-12.
10. Brenner DM, Moeller MJ, Chey WD, Schoenfeld PS. The utility of probiotics in the treatment of irritable bowel syndrome: a systematic review. Am J Gastroenterol 2009;104(4):1033-49; quiz 50.
11. Czerucka D, Piche T, Rampal P. Review article: yeast as probiotics -- Saccharomyces boulardii. Aliment Pharmacol Ther 2007;26(6):767-78.
12. Mayer EA. The neurobiology of stress and gastrointestinal disease. Gut 2000;47(6):861-9.
13. Whitehead WE, Crowell MD, Robinson JC, Heller BR, Schuster MM. Effects of stressful life events on bowel symptoms: subjects with irritable bowel syndrome compared with subjects without bowel dysfunction. Gut 1992;33(6):825-30.
14. Whorwell PJ, Altringer L, Morel J, et al. Efficacy of an encapsulated probiotic Bifidobacterium infantis 35624 in women with irritable bowel syndrome. Am J Gastroenterol 2006;101(7):1581-90.
15. Camilleri M. Peripheral mechanisms in irritable bowel syndrome. N Engl J Med 2012;367(17):1626-35.
16. Trasande L, Blustein J, Liu M, Corwin E, Cox LM, Blaser MJ. Infant antibiotic exposures and early-life body mass. Int J Obes (Lond) 2013;37(1):16-23.
17. Upadhyay V, Poroyko V, Kim TJ, et al. Lymphotoxin regulates commensal responses to enable diet-induced obesity. Nat Immunol 2012;13(10):947-53.
18. Perez-Cobas AE, Gosalbes MJ, Friedrichs A, et al. Gut microbiota disturbance during antibiotic therapy: a multi-omic approach. Gut 2012.
19. Hernandez E, Bargiela R, Diez MS, et al. Functional consequences of microbial shifts in the human gastrointestinal tract linked to antibiotic treatment and obesity. Gut Microbes 2013;4(4).
20. Everard A, Cani PD. Diabetes, obesity and gut microbiota. Best Pract Res Clin Gastroenterol 2013;27(1):73-83.
21. Everard A, Belzer C, Geurts L, et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A 2013;110(22):9066-71.