Reports
The Role of Biologics in Psoriatic Arthritis Disease Control
This report is based on medical evidence presented at sanctioned medical congress, from peer reviewed literature or opinion provided by a qualified healthcare practitioner. The consumption of the information contained within this report is intended for qualified Canadian healthcare practitioners only.
PHYSICIAN PERSPECTIVE - Viewpoint based on presentations from the Annual Meeting of the Canadian Rheumatology Association
Victoria, British Columbia / March 28-31, 2012
Reviewed and edited by:
Majed M. Khraishi, MB, BSc, FRCPC
Medical Director (Rheumatology)
Nexus Clinical Research
Clinical Professor of Medicine (Rheumatology)
Memorial University of Newfoundland
St. John’s, Newfoundland & Labrador
Introduction
Psoriatic arthritis (PsA) is a chronic inflammatory disease with a heterogeneous presentation that may include single or multiple joints with variable degrees of skin involvement. It affects up to 40% of patients with psoriasis.1 Relative to rheumatoid arthritis (RA), it has numerous distinguishing features other than the psoriatic lesions. These include a greater likelihood of inflammation in distal joints and greater likelihood of an asymmetrical pattern of joint disease. PsA is far less likely to be associated with elevated rheumatoid factor
than RA. Although once thought to have a less aggressive course than RA, more recent studies suggest that the risk of radiological progression is similar.2 In a characterization of one clinic population thought to be typical, 67% had erosive disease.3 About 20% of patients eventually develop a highly destructive and disabling form of PsA.4
Not all of the therapies approved for the treatment of rheumatoid arthritis (RA) have demonstrated efficacy in psoriatic arthritis (PsA), a potentially more challenging disease. Moreover, the relative efficacy of available therapies has been less well defined in PsA than RA, for which more multicentre studies have been conducted using disease-specific end points.
An effort to collate clinical data regarding the efficacy of current treatment options for PsA was recently undertaken by a task force of the European League Against Rheumatism (EULAR).5 The systematic literature review has provided varying levels of evidence that NSAIDs, disease-modifying antirheumatic drugs (DMARDs), glucocorticoids and tumour necrosis factor (TNF) inhibitors attenuate the signs and symptoms of PsA. The ability to slow progression of PsA has been primarily generated by studies with TNF inhibitors. The data suggest that treatment selection should be tailored to the severity of the disease course in individual patients.
The debate about the most appropriate sequence of therapies for PsA disease control runs parallel to a similar debate in the treatment of RA. Although it is reasonable to limit treatment to the most well-tolerated therapy that relieves symptoms, the potential for using
aggressive therapy early in the course of the disease to prevent progression and irreversible joint damage is also appealing, particularly for patients with rapidly advancing disease or severe involvement. Although the literature review conducted by the EULAR task force
did not attempt to provide a treatment algorithm, it did rank the quality of evidence for different treatment options across end points, providing guidance for treatment decisions.
In the EULAR task force literature review, the initial intent was to limit the analysis to double-blind, randomized controlled trials (RCTs) for each of the commonly used treatments in PsA. However, the authors permitted observational and cohort data for NSAIDs and DMARDs to be incorporated into their analysis because of the limited number of RCTs for these treatment categories. The most data and the only
level 1 evidence for efficacy were generated by studies with TNF inhibitors.
Overall, there are limited data to support the ability of NSAIDs, DMARDs and glucocorticoids to provide symptom relief in patients with PsA. Of the 2 double-blind studies with NSAIDs, an evaluation of nimesulide associated this agent with a significant reduction in pain, morning stiffness and swollen joint score at week 4. The other RCT, conducted with celecoxib, showed benefit relative to placebo at 2 weeks but no significant difference at 12 weeks, an outcome that may have been due to a high placebo response. Other non-placebo-controlled studies have reinforced the potential for symptom relief from NSAIDs, but there are no data to determine whether NSAIDs differ for efficacy in PsA.
Available Treatments
There was considerable variability in the quality of data testing DMARDs but few RCTs. For example, methotrexate (MTX) has been studied in 3 RCTs in PsA, but the total number of patients randomized was only 93. Although symptomatic improvement was associated with MTX, a case-control analysis did not show protection from radiographic progression. A larger pool of data supports the ability of sulfasalazine to control both peripheral arthritis and psoriasis, but no protection from radiographic progression was observed in the small case-control study that evaluated this outcome. Similarly, there are RCTs associating cyclosporine, leflunomide and gold salts with benefit against joint symptoms, but leflunomide was the only one of these 3 agents to be associated with benefit against psoriasis. The authors were unable to find sufficient data to judge the efficacy of azathioprine, chloroquine, penicillamine D, fumaric acid or colchicine.
According to the EULAR task force, there are no RCTs with glucocorticoids despite the evidence that these are widely used. There are numerous published review articles indicating that glucocorticoids have a role in symptom control of PsA, but the task force suggested that more data are needed, not only to judge efficacy relative to other options but also to evaluate this efficacy in the context of safety due to the many risks of chronic glucocorticoid use.
TNF Inhibitors and Protection Against Radiologic Damage
The substantial number of controlled trials with TNF inhibitors stands in juxtaposition to the more limited data on NSAIDs, DMARDs and glucocorticoids. The task force identified 11 RCTs that together included >1000 PsA patients. The studies tended to be relatively rigorous with objective measurements of activity on joint symptoms and psoriatic lesions. Some studies included evaluations of improvement on such measures as spinal symptoms, dactylitis, enthesitis and nail disease. Most of the studies evaluated the effect of these agents on radiologic progression.
In general, TNF inhibitors were associated with benefit on most of the outcomes analyzed, including improvement in global health assessment questionnaires. Most notable of the reported benefits were protection against radiologic damage at both 6 and 12 months and the consistent ability of the TNF inhibitors to provide improvement in the Psoriasis Area and Severity Index (PASI) score. Of the 9
studies that evaluated PASI, all TNF inhibitors were better than placebo at both 12 and 24 weeks.
The RCTs evaluated by the EULAR task force did not associate the TNF inhibitors with significant toxicity. However, the task force stated that studies with longer follow-up would be useful to better characterize the risk of these agents. The task force did attempt to evaluate relative efficacy among the TNF inhibitors but did not find significant differences between agents for either joint or skin manifestations.
Differences in Relative Efficacy
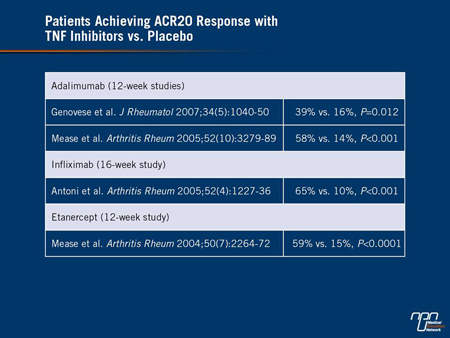
However, another detailed analysis of published TNF inhibitor studies did suggest that there might be relative efficacy differences.6 In the absence of head-to-head trials, the authors employed a mixed treatment comparison to evaluate relative efficacy of etanercept, infliximab and adalimumab, which the authors identified as the most commonly prescribed TNF inhibitors in PsA. The analysis included all RCTs with the primary outcome of American College of Rheumatology 20% (ACR20) improvement in disease parameters using standard dosages after at least 3 months on therapy. The 4 studies meeting these selection criteria entered a total of 820 patients.
While all TNF inhibitors performed significantly better than placebo (Table 1), the authors suggested that there were differences in the probability of benefit based on a Bayesian framework using pairwise comparisons. Despite the inherent limitations of crosstrial comparisons, such as potential differences in patient populations enrolled, the authors found that the odds ratios for achieving an ACR20 were higher with adalimumab relative to infliximab and higher with etanercept relative to adalimumab. Translated into clinical terms, the authors concluded that etanercept had a 71% probability of being the most efficacious for an end point of ACR20. In the absence of
controlled trials, the authors of this analysis suggested these data may be useful for guiding initial treatment choices although head-to-head trials are essential for definitive comparisons.
Table 1.
Evidence Favouring Earlier Treatment
While these data suggest that TNF inhibitors may not be interchangeable as first-line therapy for PsA, the question of when to initiate TNF inhibitors relative to other options is one of intensifying interest based on evidence that earlier treatment might provide better protection against disease progression. In a study conducted at the University of Toronto Psoriatic Arthritis Clinic, Ontario, prospectively followed patients were divided into 2 groups: those seen within 2 years of diagnosis and those seen >2 years after diagnosis.7 The first group (n=436) had less joint damage than the second group (n=641). Although the first group was younger and more likely to be treated
with DMARDs than the second, the clinical damage was lower among those with shorter disease duration, even after adjusting for age, gender, education level, clinical joint damage at first visit and treatment.
Similar findings were produced by new data presented at the Canadian Rheumatology Association (CRA) 2012 meeting.8 In this study, 196 patients managed at a PsA clinic were stratified into those with disease duration of ≥2 years and those with a shorter duration of disease. Despite a similar gender distribution and median age, those with longerduration PsA had a significantly higher number of
abnormal radiographic changes in hands, wrists and feet when compared to those with shorter duration. Although those with shorter duration had higher psoriasis scores, this group derived a greater improvement in quality of life when placed on treatment, indicating a dominant role for joint pain.
Early Manifestation of Joint Deterioration
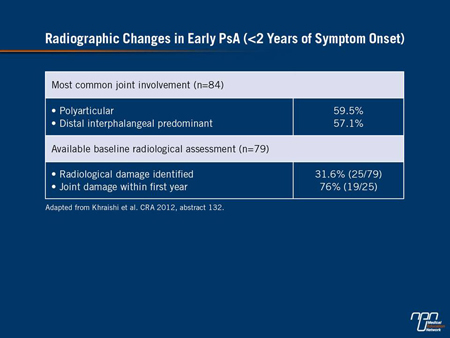
Although these findings are consistent with the premise that early treatment may be appropriate to reduce the risk of joint damage, a separate analysis by the same team of authors found that joint deterioration begins quickly.9 In the 84 patients with PsA <2 years’
duration, 32% were found to have radiologic damage. Of these, 76% had demonstrated radiologic damage within the first year. The authors noted that 59.5% of patients had polyarticular involvement and 57.1% could be characterized as having distal interphalangeal
predominant disease (Table 2). The authors of these studies employed the results to conclude that early diagnosis and management may be an important strategy for reducing disease burden.
Table 2.
A Potential for Reversing Existing Damage
While the ideal may be to initiate treatment capable of preventing joint damage early in the course of PsA, there is a small but intriguing body of evidence to suggest that TNF inhibitors may be capable of reversing existing damage. A phenomenon also reported in the treatment of RA, several case reports in PsA have now demonstrated improvement in radiographic images following initiation of a TNF
inhibitor. In one, improvements in erosion score and joint narrowing in the hands and feet of a patient was reported after the initiation of etanercept.10 In a separate analysis of radiographic studies undertaken over 3 years in several patients on longterm etanercept, repair of bone erosive changes were observed.11 Larger prospective studies are needed to further evaluate this effect.
Prospective studies are needed to demonstrate that early, aggressive therapy in PsA reduces the risk of progressing to severe disability, but this is a reasonable expectation. While it may also be reasonable to withhold TNF inhibitors until joint damage occurs, particularly if one or more of these agents prove effective in permitting bone to regenerate, it is likely that the best strategy will involve individualized care. The growing body of data indicating an acceptable degree of safety and tolerability of TNF inhibitors in PsA will facilitate their use in appropriate candidates.
Summary
Due to the limited number of RCTs with NSAIDs and DMARDs, it is unclear when to switch from one of these agents in patients with progressive PsA. While managing PsA with NSAIDs or DMARDs may be a reasonable approach for individuals with mild disease, the best evidence for disease control, particularly at the radiologic level, is with TNF inhibitors. Early treatment with TNF inhibitors has particular potential to preserve joint structures and reduce the risk of progressive disability. Efforts to develop firm guidelines to define the role of first-line TNF inhibitor therapy are ongoing.
References
1. Khraishi et al. High prevalence of psoriatic arthritis in a cohort of patients with psoriasis seen in a dermatology practice. J Cutan Med Surg 2012;16(2):122-7.
2. Rahman et al. Comparison of radiological severity in psoriatic arthritis and rheumatoid arthritis. J Rheumatol 2001;28(5):1041-4.
3. Torre Alonso et al. Psoriatic arthritis (PA): a clinical, immunological and radiological study of 180 patients. Rheumatol 1991;30(4):245-50.
4. Gladman et al. Psoriatic arthritis: epidemiology, clinical features, course, and outcome. Ann Rheum Dis 2005;64 (suppl 2):ii14-7.
5. Ash et al. A systematic literature review of drug therapies for the treatment of psoriatic arthritis: current evidence and meta-analysis informing the EULAR recommendations for the management of psoriatic arthritis. Ann Rheum Dis 2012;71(3):319-26.
6. Migliore et al. Indirect comparison of etanercept, infliximab, and adalumimab for psoriatic arthritis: mixed treatment comparison using placebo as common comparator. Clin Rheumatol 2012;31(1):133-7.
7. Gladman et al. Do patients with psoriatic arthritis who present early fare better than those presenting later in the disease? Ann Rheum Dis 2011;70(12):2152-4.
8. Khraishi et al. Comparative analysis of the cohorts with early and established psoriatic arthritis (PsA). Canadian Rheumatology Association, Victoria, BC, 2012, abstract 133.
9. Khraishi et al. Early radiographic changes in patients with psoriatic arthritis. Canadian Rheumatology Association, Victoria, BC, 2012, abstract 132.
10. Eder L, Chandran V, Gladman DD. Repair of radiographic joint damage following treatment with etanercept in psoriatic arthritis is demonstrable by 3 radiographic methods. J Rheumatol 2011;38(6):1066-70.
11. Garcia-Valladares I, Cuchacovich R, Espinoza LR. Psoriatic arthritis: radiographic joint repair following etanercept therapy. J Rheumatol 2012;39(1):185; author reply 186.