Reports
Treatment and Prevention of Venous Thrombosis in Cancer
This report is based on medical evidence presented at sanctioned medical congress, from peer reviewed literature or opinion provided by a qualified healthcare practitioner. The consumption of the information contained within this report is intended for qualified Canadian healthcare practitioners only.
MEDICAL FRONTIERS - 6th International Conference on Thrombosis and Hemostasis Issues in Cancer
Bergamo, Italy / April 20-22, 2012
Bergamo - Cancer is a hypercoagulable state where malignancy heightens thrombotic risk throughout the course of active disease, especially during metastases. Anti-angiogenic chemotherapy agents can significantly increase embolic risk, as well as surgery and central venous catheters. Never benign, when venous thromboembolism (VTE) occurs, it negatively affects quality of life for cancer patients and may prove fatal. Low molecular weight heparins (LMWH) are widely recommended as the most effective strategy for the treatment and prevention of VTE in the setting of malignancy. Whether VTE can be prevented in the primary setting is still debated but there is some evidence that LMWHs extend survival by preventing VTE in patients with less advanced cancer and may improve prognosis if used early enough in cancers with the highest thrombotic risk.
Chief Medical Editor: Dr. Léna Coïc, Montréal, Quebec
Cancers and Thrombotic Risk
The risk of venous thromboembolism (VTE) varies according to the type of cancer where hematologic, lung and gastrointestinal (GI) cancers probably confer the highest thrombotic risk. Cancer of the pancreas, prostate and colon have all been associated with a prothrombotic state characterized by clotting factor abnormalities as well. VTE is also more likely to occur in patients with advanced cancer and metastatic disease. Thrombotic risk also peaks at several points along the cancer journey. “VTE is the most common complication we see in cancer and the second most common cause of death in patients with active cancer, second only to disease progression,” Dr. Alexander Cohen, King’s College Hospital, London, UK, told delegates here at ICTHIC.
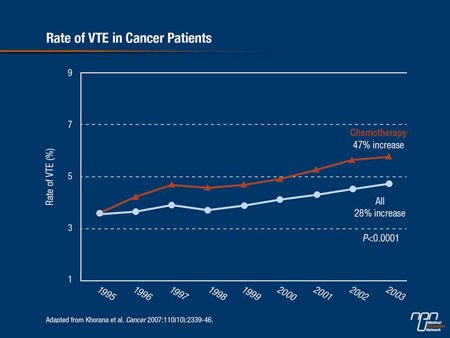
The incidence of VTE is also increasing in cancer patients. From 1995 to 2003, the rate of VTE in cancer patients receiving chemotherapy increased by 47% (Cancer 2007;110(10):2339-46) (Figure 1). Others have also reported finding radiological evidence of VTE in 50% of hospice patients with advanced cancer, suggesting that it is significantly underdiagnosed in many cancer patients—for example, in those with lung cancer where symptoms of a pulmonary embolism (PE) mimic those of lung cancer. “As patients become less well, they become less ambulant and as tumour burden increases, so too do procoagulant abilities,” explained Dr. Simon Noble, Cardiff University, UK. Then there is a small number of lung cancer patient candidates for surgery and chemotherapy until disease progresses, after which there may be no further therapy but comorbidities increase (infections, heart failure), further limiting patient mobility.
“It is said that 50% of cancer patients die with evidence of thrombosis, and mortality is very high in cancer patients who develop VTE so the burden of thrombotic complications in cancer patients is huge,” stated Dr. Agnes Lee, Associate Professor of Medicine, University of British Columbia, and Director, Thrombosis Program, Vancouver General Hospital. “If we were more aggressive with VTE prevention, we could have an impact on mortality.”
LMWH Evidence
All major guidelines continue to recommend monotherapy with low molecular-weight heparins (LMWHs) as the preferred treatment strategy for cancer-associated thrombosis. These recommendations were based on results from 3 randomized controlled trials (RCTs) in which 1 of 3 different LMWHs—dalteparin, enoxaparin and tinzaparin—was compared with warfarin in patients with symptomatic proximal deep-vein thrombosis (DVT) or pulmonary embolism (PE).
Figure 1.
In the CLOT (Randomized Comparison of Low-Molecular-Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer) trial (N Engl J Med 2003;349:146-53), dalteparin was given at a daily dose of 200 IU/kg for the first month, followed by 75% to 83% of the same dose for 5 months. In the other 2 studies, the LMWH was given for only 3 months. “All of these trials consistently showed that LMWH is better than warfarin in reducing the risk of recurrent VTE,” Dr. Lee reported.
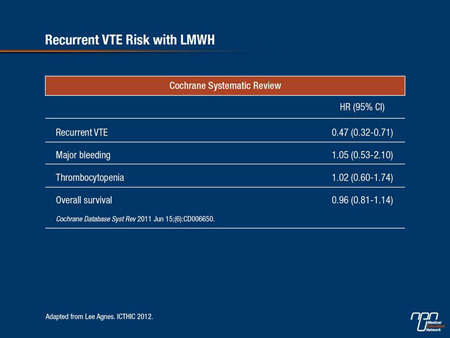
In CLOT, dalteparin reduced the risk of symptomatic recurrent thrombosis by 52% relative to warfarin and there was no difference in major bleeding between the 2 treatment arms. A Cochrane review combining results of these RCTs found a 53% reduction in the incidence of VTE in cancer patients—virtually identical to what was shown in the CLOT trial (Cochrane Database Syst Rev 2011;CD006650) (Table 1).
Suggested Management
At present, the optimal duration of anticoagulant therapy in cancer-associated thrombosis is not known. Expert guidelines indicate that physicians should continue therapy for 3 to 6 months and for as long as the cancer is active and patients are receiving chemotherapy. Beyond this period, “indefinite” therapy is recommended in patients with known metastases because of their high VTE risk.
“We also need to tailor treatment to patient needs,” Dr. Lee added. Treatment with warfarin has inconveniences, including the frequent need for laboratory monitoring and the necessity of doing venipuncture and phlebotomy to carry out INR monitoring, as well as many drug interactions and dietary restrictions.
Conversely, LMWHs are more costly than warfarin and require daily subcutaneous (s.c.) injections. If patients develop a recurrent VTE while on warfarin, they should be switched to an LMWH; if they fail on an LMWH, the dose should be increased 20 to 25% and patients reassessed in 5 to 7 days for symptomatic improvement.
The use of inferior vena cava filters is common in patients with VTE in whom anticoagulation is contraindicated, but there is no biological reason why filters should work in any patient because they do not suppress coagulation. “My recommendation is not to put these filters in, particularly in cancer patients who are hypercoagulable,” Dr. Lee suggested.
Table 1.
Post-surgical VTE Prevention
Surgery remains the mainstay for the management of solid tumour malignancies, with over 80% of patients undergoing surgery at some point in their disease. For many, early surgical intervention is offered with intent to cure or at least achieve prolonged palliation of symptoms. As such, “prevention of a potentially fatal complication such as VTE must be a key priority,” Dr. Ajay Kakkar, Professor of Surgery, University College, and Director, Thrombosis Research Institute, London, UK, told delegates.
Guidelines recommend patients receive preoperative s.c. heparin, followed by continuous administration of anticoagulation starting 12 to 24 hours after surgery. Antithrombotic prophylaxis should be continued for at least 7 to 10 days post-operatively. A Cochrane review that included cancer patients undergoing abdominal, pelvic or thoracic surgery showed that extended prophylaxis following cancer surgery reduced the rate of DVT by over 50% and that of proximal DVT by 75% compared with prophylaxis given for 7 to 10 days (Cochrane Database Syst Rev 2009;CD004318).
More recently, a double-blind trial in patients undergoing abdominal or pelvic surgery for cancer similarly found that extended prophylaxis with the LMWH bemiparin decreased the rate of major VTE without increasing bleeding complications (J Thromb Haemost 2010;8:1223-9). In response to this, several guidelines now recommend extended prophylaxis for all patients undergoing elective cancer surgery, although some, such as ASCO, recommend extended prophylaxis only for cancer patients at high thrombotic risk who are undergoing major abdominal or pelvic surgery.
“We also know that to achieve a benefit from in-hospital prophylaxis, cancer surgical patients need a higher dose of an LMWH than a benign surgical population,” as Dr. Kakkar noted, citing a reduction in VTE in 1 study from 15% to 8% when a high dose of dalteparin (5000 units) was used compared to a 2500-unit dose.
Primary Thrombotic Prevention
If it is clear that LMWHs are the preferred agents for the treatment and secondary prevention of VTE, their ability to prevent primary thrombotic events in ambulatory patients undergoing chemotherapy is still being debated. As discussed by a number of speakers, specific chemotherapy regimens, especially those containing angiogenesis inhibitors, are associated with an increased risk of VTE. In the setting of certain hematological malignancies, notably multiple myeloma (MM), VTE risk increases dramatically due to the multi-agent regimen used to treat the malignancy, especially if it contains thalidomide or lenalidomide.
Because of the high incidence of VTE in this patient population, “thromboprophylaxis has been introduced mainly during induction treatment,” noted Dr. Frank Leebeek, Erasmus University Medical Center, Rotterdam, The Netherlands. The optimal antithrombotic regimen in MM is not clear, but evidence suggests that the use of prophylactic LMWH, full-intensity warfarin with a target INR of 2-3 or fixed dose warfarin all reduce the risk of thromboembolic complications in this disease.
Recently, Prof. Giancarlo Agnelli, University of Perugia, Italy, and colleagues reported that with a median treatment of 3.5 months, the LMWH semuloparin led to a significant 64% relative risk reduction in thrombotic outcomes compared with placebo (N Engl J Med 2012;366:601-9). “This effect was consistent across different types of cancer,” he reported, “and against the different components of the end point including symptomatic DVT, any PE and non-fatal PE.” Bleeding risk was also only about 1% in both treatment arms.
A trend towards efficacy using certoparin prophylaxis was also shown in advanced non-small cell lung cancer (NSCLC) patients in TOPIC-2, where the risk of VTE at 8.3% in placebo controls was very high, a sign that NSCLC is highly thrombotic (Clin Appl Thromb Hemost 2012;18:159-65). When data on NSCLC patients from both PROTECHT and TOPIC-2 are combined, “we are looking at the number-needed-to-treat (31) vs. the number-needed-to-harm (125) to prevent 1 VTE and I think these numbers are very impressive. The cost of treating VTE in cancer is also significantly more than the cost of preventing it in the same patients,” Dr. Noble told delegates.
Potential Anticancer Effect
It has been suggested that LMWHs may improve survival by preventing a potentially fatal VTE and also by direct anti-cancer effects. Preclinical evidence indicates that the hemostatic system is very closely linked to tumour growth and proliferation, thrombin serving as a growth factor for angiogenesis.
LMWHs can also decrease seeding from the primary tumour and influence multiple steps along the metastatic cascade, decreasing inflammation, angiogenesis and endothelial cell adhesion, among other anti-metastatic effects. In the FAMOUS study, although there was no difference in survival at 1 year in patients with advanced solid tumours randomized to dalteparin vs. placebo, a post-hoc analysis survival at 2 and 3 years showed that there appeared to be a survival benefit in patients who had better prognosis tumours at study entry and who were still alive after 17 months (J Clin Oncol 2004;22(10):1944-48).
Moreover, as Dr. Noble pointed out, “The LMWH would have been stopped at 12 months so this survival benefit couldn’t be explained by thromboprophylaxis. One of the explanations for this survival benefit is that there may be some disease modification going on with the LMWH.” Similarly, Altinbas et al. (J Thromb Haemost 2004;8:1266-71) found that the addition of dalteparin to chemotherapy improved response rates to chemotherapy at 69% vs. 42% for chemotherapy alone and contributed to an improved median overall survival of 13 months vs. 8 months.
Somewhat unique to this study, a similar improvement in survival with additional LMWH to chemotherapy was seen in patients with both limited and extensive disease. As explained by Prof. Ismail Elalamy, Paris VI University, France, the development of blood clots protects tumour cells from both the immune system and chemotoxic drugs. By preventing thrombus formation, “LMWHs facilitate access of chemotherapy to tumour cells,” he indicated. “Whether in the arterial or venous milieu, thrombus is the enemy and if you use LMWH as early as possible in less advanced disease, you can observe a reduction in thrombotic events and a gain in survival.”
The ongoing phase III FRAGMATIC study, designed to mimic clinical practice as closely as possible, promises to shed more light on whether LMWHs exert anti-cancer effects. Researchers are evaluating the effects of dalteparin on top of best standard of care in lung cancer patients (both small-cell and NSCLC) at any stage. Participating patients are receiving best individualized care, consisting of surgery, chemotherapy, radiation therapy or supportive care. Patients are randomized to receive dalteparin or placebo for 6 months. FRAGMATIC primary end point is overall survival, and secondary end points include VTE rates, quality of life, toxicity and metastasis-free survival. Investigators are also performing a health economic analysis to determine whether LMWH use in this particular cancer setting reduces overall costs to the health care system.
“Imagine for a moment,” suggested Dr. Noble, “that if no survival benefit is shown, but the overall cost to the health care system is reduced, it would still be a very important [finding]. We closed recruitment in December 2011, recruited 2203 patients and hope to have the preliminary results available in early 2013.” Surmising whether or not LMWHs improve survival in lung cancer, Dr. Noble concluded, “There is a sufficient number of well-run, good-quality studies that are ongoing, at least in lung cancer, that we should have an answer in the near future.”
Questions and Answers
The following question-and-answer session was conducted with Dr. Agnes Lee, Associate Professor of Medicine,
University of British Columbia, and Director, Thrombosis Program, Vancouver General Hospital.
Q: Are there any reasons to favour 1 LMWH over another for the initial and long-term treatment of VTE in cancer patients as is recommended?
A: The LMWH with the best evidence is dalteparin. So if you have a choice, then it makes sense to use this agent because it has the most robust evidence. It is also the only LMWH that has regulatory approval for the extended treatment of VTE in cancer patients.
Q: Do you believe that the LMWHs have potential anti-cancer effects?
A: I do. But LMWHs are not going to have an effect on all cancers and just like chemotherapy, they will have very little impact at the end of life, when patients have advanced disease. So we have to introduce them early and I think we should be aiming for those cancers that show a higher incidence of thrombosis, as clearly there is an interplay between activation of coagulation and tumour growth and it makes sense to target tumours with a high thrombosis risk.
Q: What do you feel physicians must keep in mind when considering the use of LMWHs for VTE treatment and prevention?
A: We need to pay attention and prevent thrombotic events. If we don’t educate our patients about the signs and symptoms of thrombosis, we are going to miss the boat and then we will be dealing with the burden of a thrombotic event. So we need to educate patients about thrombosis because nobody ever mentions thrombosis as a potential complication in cancer therapy.