Reports
Weighing Benefits of SGLT2 Inhibition in Type 2 Diabetes Mellitus
This report is based on medical evidence presented at sanctioned medical congress, from peer reviewed literature or opinion provided by a qualified healthcare practitioner. The consumption of the information contained within this report is intended for qualified Canadian healthcare practitioners only.
ABSTRACTS in PERSPECTIVE - Based on presentations from the 48th Annual Meeting of the European Association for the Study of Diabetes (EASD)
Berlin, Germany / October 1-5, 2012
EDITORIAL OVERVIEW:
David C. W. Lau, MD, PhD, FRCPC
Editor-in-Chief, Canadian Journal of Diabetes
Professor of Medicine, Biochemistry & Molecular Biology
Julia McFarlane Diabetes Research Centre
University of Calgary
Calgary, Alberta
Ronald M. Goldenberg, MD, FRCPC, FACE
Consultant Endocrinologist
North York General Hospital
Medical Co-Director
LMC Diabetes and Endocrinology
Thornhill, Ontario
Treatment with many currently available antihyperglycemic agents is commonly limited by weight gain and hypoglycemia. Most of these agents are dependent on insulin secretion and/or insulin action. The kidney has been considered an alternative target for therapeutic intervention in diabetes because of its role in the reabsorption of filtered glucose via sodium glucose cotransporters (SGLTs). SGLT2, which occurs predominantly in the earlier segments of the proximal tubule, is responsible for reabsorption of >90% of the glucose filtered and has become the focus of interest for development of a new class of oral agents with a completely novel, insulin-independent mechanism of action for reducing blood glucose.
A number of SGLT2 inhibitors are currently at different phases of development. The focus of several scientific sessions in Berlin included dapagliflozin and canagliflozin, both of which have already been submitted for regulatory approval in the US and the European Union. Others, such as empagliflozin, ipragliflozin and tofogliflozin, are in phase III trials.
Clinical studies have shown that SGLT2 inhibitors produce dose-dependent reduction in HbA1c, with a low potential for hypoglycemia as monotherapy or in combination therapy. They are also associated with sustained reductions in body weight, initially due to the osmotic diuresis followed by urinary caloric loss over time.
These findings were highlighted in a meta-analysis presented at the meeting. Data extracted from 39 randomized controlled trials compared any SGLT2 inhibitor with placebo or any other antihyperglycemic medication, as monotherapy or as add-on therapy, over ≥12 weeks. SGLT2 inhibitors were shown to be associated with a significantly greater decline in HbA1c vs. placebo (-0.7%) and as add-on treatment vs. active comparator (-0.2%) and with a similar efficacy to metformin as first-line therapy. All SGLT2 inhibitor regimens produced greater reductions in body weight and in blood pressure (BP), due to the diuretic/natriuretic effect. The risk for hypoglycemia was raised slightly compared with placebo (RR 1.10), but was lower than active comparators (RR 0.36).
The most frequent adverse events (AEs) consistently reported with SGLT2 inhibitors were dose-dependent increases in rates of genital tract infections (GTIs), especially in women, due to the pharmacological induction of glucosuria. The absolute numbers were small, however, and the infections usually resolved with standard therapies. Urinary tract infections (UTIs) were less frequent, but increased vs. placebo.
SGLT2 Inhibitor Efficacy and Safety in Clinical Trials
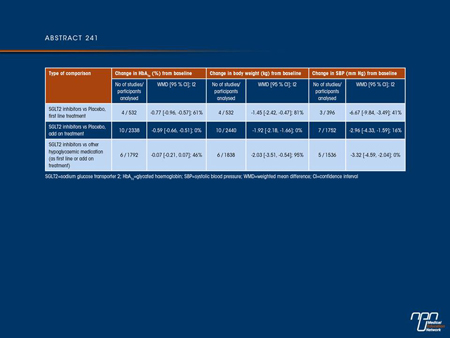
Data supporting the efficacy and safety of canagliflozin 100 or 300 mg as add-on to insulin therapy were reported from the ongoing CANVAS (Canagliflozin Cardiovascular Assessment Study). Patients enrolled in CANVAS had elevated cardiovascular (CV) risk due to documented symptomatic atherosclerotic disease (age ≥30 years) or ≥2 CV risk factors (age ≥50 years). A prespecified 18-week substudy carried out in 1718 participants already receiving insulin ≥30 IU/day showed significant reductions in HbA1c (-0.65 to -0.73%), FPG (-1.3 to -1.6 mmol/L), body weight (-1.8 kg to -2.4 kg) and systolic BP (SBP) (-2.6 to -4.4 mm Hg) (all P<0.001 vs. placebo). A higher proportion of patients on canagliflozin reached HbA1c <7.0%. The SGLT2 inhibitor was associated with a significant increase in HDL-C and a slight increase in LDL-C with the 300 mg dose. The mechanism for the changes in lipids remains unknown. The agent was well tolerated except for higher rates of GTIs and osmotic diuresis-related AEs. The incidence of hypoglycemia was low across all groups.
Two 52-week phase III studies demonstrating the efficacy of canagliflozin 100 and 300 mg as add-on to metformin were presented, including the first comparison with a dipeptidylpeptidase 4 (DPP-4) inhibitor. In the 755 patient study, canagliflozin 300 mg was associated with greater glycemic control compared with sitagliptin 100 mg.Mean HbA1c was reduced by -1.03% from baseline compared to -0.66% with sitagliptin (P<0.001). A greater proportion of canagliflozin-treated subjects achieved HbA1c targets. Other significant differences included FPG (-1.7 mmol/L vs. -0.3 mmol/L), body weight (-2.3 kg vs. 0.1 kg) and BP (-5.1 mm Hg vs. 0.9 mm Hg), respectively. Small absolute increases in HDL-C and LDL-C were greater with canagliflozin. Rates of AEs were similar on the 2 treatment arms except for higher rates of GTIs with canagliflozin.
In a study comparing canagliflozin (100 and 300 mg) with glimepiride (titrated up to 6 or 8 mg/day) in 1450 patients over 52 weeks, canagliflozin treatment was associated with a consistently lower compared with an increased HbA1c with glimepiride after week 18. The 100 and 300 mg doses provided greater body weight loss (-3.7 and -4.0 kg vs. 0.7 kg) and fewer hypoglycemic episodes (5.6% and 4.9% vs. 34.2% of patients) compared with glimepiride. They also produced improvements in FPG, SBP, triglycerides and HDL-C. More patients on glimepiride required rescue therapy (10.6%) than with canagliflozin 100 mg (6.6%) or 300 mg (4.9%). The overall incidence of AEs was similar (66%) in all treatment groups.
An analysis of 24-week data from 5 clinical trials showed that dapagliflozin 10 mg/day, as monotherapy or add-on to metformin, glimepiride, pioglitazone or insulin consistently reduced HbA1c in a dose-dependent manner, by -0.54% to -0.68% (P<0.0001). Reductions were also seen in FPG and PPG. Body weight was significantly reduced vs. placebo as add-on to metformin, glimepiride, pioglitazone or insulin (all P<0.0001) and by up to 1 kg as monotherapy. The SGLT2 inhibitor was generally well tolerated. Proportions of patients reporting hypoglycemia were low when used as monotherapy or combined with metformin or pioglitazone, but higher when combined with glimepiride or insulin. Events suggestive of GTIs were consistently reported more frequently with dapagliflozin across all studies. UTI rates were higher with monotherapy and as add-on to insulin but balanced across treatments in the remaining studies.
Similar rates of infections were reported from an analysis of pooled data extending up to 2 years from 12 Phase IIb/III double-blind placebo-controlled trials. In addition, renal impairment and failure was rare and equally distributed among all groups including placebo (0.9-1.4% vs. 0.9%) and volume depletion AEs (hypotension/dehydration/hypovolemia) were slightly more frequent (0.6-1.2% vs. 0.4%), but all were non-serious. Normal and elevated liver function tests were similar with dapagliflozin and placebo. No unacceptable increased risk in CV events was identified and incidences of malignant and unspecified tumours were similar for dapagliflozin vs. comparators.
Weight Reduction
Long-term use of an SGLT2 inhibitor in overweight/obese patients with T2DM resulted in clinically meaningful reductions in HbA1c and sustained body weight loss according to an analysis of data from 4 extended phase III trials with dapagliflozin. The trials compared dapagliflozin 10 mg daily as monotherapy with placebo or comparator drugs in patients with different background treatment and at different stages of disease. Dapagliflozin treatment was associated with significant reductions in total body weight at the end of the 24-week studies (-1.72 to -3.38 kg) and sustained over 2 years (-1.97 to -3.70 kg).
Body composition analysis in 374 patients from 2 phase III studies showed that the weight reduction associated with SGLT2 inhibition occurs mainly through loss of fat. In both studies, canagliflozin 100 and 300 mg was associated with significant body weight reduction, either at 52 weeks vs. glimepiride in subjects on background metformin or at 26 weeks vs. placebo in patients on other antihyperglycemic regimens (study 2). Dual-energy X-ray absorptiometry revealed that loss of fat mass accounted for approximately two-thirds of the overall body weight reduction with canagliflozin, with greater reductions in per cent total fat compared with glimepiride or placebo. Notably, analysis of abdominal fat by computed tomography in study 1 showed a slightly greater fat loss from visceral than subcutaneous adipose tissue.
According to results from an observational Swedish study in a cohort of 8486 subjects with no history of previous CV disease or cancer, those subjects, whose BMI increased over the year following newly diagnosed diabetes, were found to be at 63% increased risk for CV mortality and 34% for all-cause mortality compared with subjects with stable BMIs. The patients, who were identified at primary care centres between 1999 and 2008, were followed for a median of 4.6 years. Compared with the stable BMI group, subjects with increased BMI were found to be younger and more highly educated, with lower HbA1c, higher HDL-C and higher creatinine levels and less angina pectoris.
Use in Patients with Renal Impairment
Since SGLT2 inhibitors act by inhibiting the reabsorption of filtered glucose, it was reasoned that these agents would not be effective in patients with diabetes and renal impairment. However, a phase III study showed that canagliflozin was effective and well tolerated in patients with moderate renal impairment (eGFR ≥30 and <50 mL/min/1.73 m2). In 269 patients treated with canagliflozin 100 or 300 mg for 26 weeks, HbA1c was significantly decreased by -0.33% and -0.44%, respectively, compared with placebo (P<0.05 and P<0.001). Although these reductions were lower than in individuals with normal renal function, they were viewed as clinically significant. Improvements were also seen in FPG, body weight and SBP compared with placebo. AEs related to osmotic diuresis and reduced intravascular volume were slightly higher with the 300 mg dose. Both doses were associated with transient decreases in renal function parameters that attenuated over the study period. There was no evidence of renal injury with canagliflozin treatment using urinary albumin excretion as a marker for glomerular/tubule injury.
Summary
SGLT2 inhibitors offer a novel, insulin-independent approach for the control of hyperglycemia without incurring hypoglycemia. They can be used as monotherapy or in combination therapy with insulin or other antihyperglycemic agents. They also represent the first class of oral antihyperglycemic agents associated with sustained modest body weight loss, as well as mild BP lowering. The most frequent AEs, GTIs, are mild to moderate and resolve with standard therapy. Long-term studies of CV benefit:risk are under way with these agents.
ABSTRACT 421
Systematic review and meta-analysis of the efficacy and safety of SGLT2 inhibitors in patients with type 2 diabetes mellitus
A. Tsapas, D. Vassilakou, E. Athanasiadou, T. Karagiannis, D.R. Matthews
Background and aims: Sodium-glucose co-transporter 2 (SGLT2) inhibitors are a novel class of oral antihyperglycaemic drugs that reduce renal glucose reabsorption. Their therapeutic role in type 2 diabetes has been tested in clinical trials. We performed a systematic review and meta-analyses to assess their efficacy and safety versus placebo or other antidiabetic medications.
Materials and methods: We searched Medline, Embase and the Cochrane Library, without any language restrictions, for randomised trials of more than 12 weeks duration that compared an SGLT2 inhibitor with placebo or any other hypoglycaemic medication for type 2 diabetes mellitus. Additionally, we searched websites of pharmaceutical companies, public registries of clinical trials and abstracts of major scientific meetings. Our primary outcome was glycaemic efficacy assessed by the change from baseline in HbA1c. Secondary
efficacy outcomes included changes in body weight and systolic blood pressure, and percentage of patients achieving HbA1c<7%. Safety outcomes included incidence of any hypoglycaemia, adverse events of special interest (urinary and genital tract infections), discontinuation rate and overall cardiovascular morbidity. We conducted meta-analyses using an inverse variance random effects model. Heterogeneity was assessed by the I2 statistic. Risk of bias was assessed with the Cochrane risk of bias tool.
Results: Our search identified 21 eligible trials. Sixteen studies (duration 12-104 weeks, 5030 patients) provided adequate data and were used in our meta-analyses. SGLT2 inhibitors were associated with a greater decline in HbA1c compared with placebo (Table). Risk for hypoglycaemia was similar between SGLT2 inhibitors and placebo (risk ratio [RR] 1.10, 95% confidence interval [CI] 0.92 to 1.30) but smaller compared to active comparators (RR 0.24, 95% CI 0.06 to 0.98). No difference in the incidence of urinary tract infections was
evident between SGLT2 inhibitors and placebo (RR 1.26, 95% CI 0.94 to 1.70). However, SGLT2 inhibitors were associated with an increased risk for urinary tract infections compared with active comparators (RR 1.51, 95% CI 1.08 to 2.09), and for genital tract infections compared both with placebo (RR 3.28, 95% CI 2.19 to 4.90) or other hypoglycaemic medications (RR 4.57, 95% CI 2.80 to 7.45).
Conclusions: Existing data support that SGLT2 inhibitors have a favourable effect on HbA1c, weight, systolic blood pressure and incidence of hypoglycaemia. However, they are associated with a significantly increased risk for urinary and genital tract infections.
Commentary on abstract 241
The results of this meta-analysis of studies suggest that, as a class, SGLT2 inhibitors are associated with intermediate to high efficacy in reducing HbA1c with low risk of hypoglycemia, with weight loss and a small reduction in BP. The major side effects reported with these agents were urinary and genital tract infections. The strengths of this meta-analysis were that it was comprehensive and included the latest data from unpublished as well as published studies, including extension data. Heterogeneity was assessed and the results were determined to be consistent across studies. However, many of the studies included were reported only in the form of abstracts and only a small number compared an SGLT2 inhibitor with an active comparator. No examination was made of changes in lipids or rare
adverse events such as malignancies.
Questions and answers with Dr. Ronald Goldenberg and Dr. David Lau
Q: Does reduction in renal glucose reabsorption offer clinical advantages over other antihyperglycemic medications in patients with T2DM?
A: The SGLT2 inhibitors are unique in that their action is independent of insulin secretion or action, so they are effective at any stage of T2DM, and can be used in conjunction with other antihyperglycemic agents. They will be the first class of oral antihyperglycemic agents to be associated with significant weight loss. Since over 90% of patients with T2DM are either overweight or obese, this will be quite beneficial to our patients. The efficacy of SGLT2 inhibitors in glucose lowering without causing hypoglycemia, and the modest but
sustained body weight loss are important advantages over other antihyperglycemic agents.
Q: Is the increased risk of genital or urinary tract infections indicative of a class effect?
A: Clinical studies suggest that the genitourinary tract infections (GUI) are indeed a class effect attributable to increased glycosuria, an ubiquitous property of SGLT2 inhibitors.
Q: How might one prevent the development of genital or urinary tract infections when treated with SGLT2 inhibitors?
A: Ask your patients about genitourinary tract symptoms and then test and treat as required. In general, people with diabetes have increased frequencies of GUI and it is important to monitor for symptoms and screen for GUI.
Q: Which patients with T2DM could be considered for monotherapy with SGLT inhibitors?
A: Patients who are intolerant to or have a contraindication to the use of metformin and whose HbA1c levels are generally ≤8%.
ABSTRACT 764
Efficacy and safety of canagliflozin (CANA), an inhibitor of sodium glucose co-transporter 2 (SGLT2), added-on to insulin therapy +/- oral agents in type 2 diabetes
D. Matthews, G. Fulcher, V. Perkovic, D. de Zeeuw, K.W. Mahaffey, J. Rosenstock, M. Davies, G. Capuano, M. Desai, W. Shaw, F. Vercruysse, G. Meininger, B. Neal
Background and aims: Inhibition of SGLT2 is a novel modality of treatment for patients with T2D. We report here a pre-specified substudy of the canagliflozin Cardiovascular Assessment Study (CANVAS) defining the efficacy, safety and tolerability of CANA in participants with T2D on insulin.
Materials and methods: CANVAS is a double-blind, placebo (PBO)-controlled study that randomised 4,330 individuals at elevated cardiovascular risk to the addition of PBO, CANA 100 or 300 mg, once daily, to stable ongoing diabetes therapy. In the subgroup using insulin (≥30 units/d), there were 565, 566 and 587 individuals, respectively. The primary endpoint for this sub-study was the change from baseline in HbA1c at 18 weeks follow-up while the insulin doses were kept unchanged as per protocol. Major vascular outcomes
remained blinded.
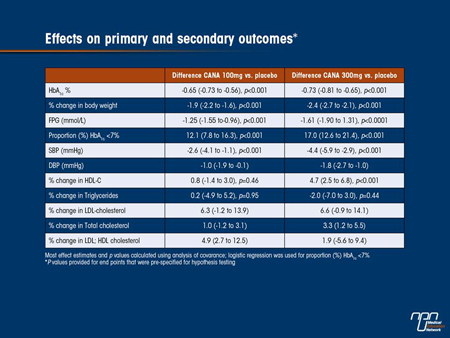
Results: Baseline demographics demonstrated no imbalances; 67% of participants were male and 59% had a history of a prior vascular event. Mean baseline age was 63 y, HbA1c 8.3%, BMI 33.8 kg/m2, body weight 97.0 kg, fasting plasma glucose 9.4 mmol/L, total cholesterol 4.3 mmol/L and blood pressure 138/76 mm Hg. Mean daily insulin dose at baseline was 83 IU with most using basal/bolus insulin regimens and a median duration of diabetes of 15 y. Compared to PBO both doses of CANA significantly improved measures of
glycaemia (Table). Adverse events (AEs) were reported for the CANA 100 mg, CANA 300 mg and PBO groups in 63%, 65% and 59% of participants and serious AEs in 5.5%, 4.9% and 6.4% respectively. Incidence of AEs leading to discontinuation was greater with CANA 300 mg (5.3%) compared to CANA 100 mg or PBO (both 1.9%). Male and female genital fungal infections were more common with CANA 100 mg (F:11.8%, M:4.0%) and 300 mg (F:9.9%, M:8.3%) compared to PBO (F:2.2%, M:0.5%) as were AEs of pollakiuria and hypotension with CANA 100 mg (3.7% and 1.2%, respectively) and CANA 300 mg (5.6% and 2.6%, respectively), compared to PBO (0.5% and 0%, respectively). A slightly higher incidence of UTI was seen with CANA 300 mg (3.4%) relative to CANA 100 mg and PBO (2.3% and 2.1%, respectively). Incidence of hypoglycaemia was higher with CANA 100 mg (49%) and CANA 300 mg (48%) than PBO (37%).
Conclusions: CANA added to stable insulin therapy improved glycaemic control and produced significant improvements in a number of efficacy parameters important in the management of T2D with effects of the 300 mg dose numerically greater than the 100 mg dose. CANA was generally well tolerated, and associated with a greater frequency of genital fungal infections and slightly higher risk of hypoglycaemia.
Commentary on abstract 764
This prespecified substudy of CANVAS (Canagliflozin Cardiovascular Assessment Study) demonstrated the efficacy and safety of canagliflozin combined with insulin in patients with a history or at high risk of CV disease. These patients were already on insulin ≥30 IU/day (mean dose 83 IU/day, mainly basal/bolus regimens) as well as other oral antihyperglycemic agents. Their insulin doses were
kept unchanged as per protocol. The patients in this substudy were older than usual for this type of study (mean age 62 years), had a high BMI (mean 33.8) and a long duration of diabetes (mean 16 years), and had already been on insulin therapy for about 7 years. This was a short-term study of only 18 weeks, but over this time there were significant reductions in HbA1c with both doses of canagliflozin, accompanied by reductions in fasting glucose, weight loss, SBP and a significant increase in HDL-C. More patients on canagliflozin reached HbA1c <7.0% and both doses were associated with a good tolerability profile. This substudy did not compromise the CV outcome data for the main CANVAS study, which remains blinded and is in the follow-up phase.
Questions and answers with Dr. Ronald Goldenberg and Dr. David Lau
Q: Typically what per cent of patients with T2DM in a family practice are on insulin?
A: Insulin tends to be underused in the management of T2DM. The Diabetes in Canada Evaluation study from 2005 indicated that only 12% of patients with T2DM were taking insulin. The DRIVE registry indicated that 15% of patients with T2DM were treated with insulin.
Q: Does the data presented look reassuring in terms of the safety of adding canagliflozin to patients on insulin?
A: The CANVAS substudy demonstrates that canagliflozin added to insulin therapy is a safe and effective way to manage insulin-treated patients with T2DM.
Q: Will SGLT2 inhibitors allow for a reduction in insulin dose with improvement in glycemic control when combined with insulin therapy?
A: In a dapagliflozin trial, patients took about 7U/day less insulin when dapagliflozin was combined with insulin compared to insulin alone, with about a 0.6% further lowering in HbA1c.
ABSTRACT 243
Efficacy and safety of canagliflozin, a sodium glucose co-transporter 2 inhibitor, compared with sitagliptin in patients with type 2 diabetes on metformin plus sulphonylurea
G. Schernthaner, J. Gross, M. Fu, J. Yee, M. Kawaguchi, W. Canovatchel, G. Meininger
Background and aims: Canagliflozin (CANA) is an inhibitor of the sodium glucose co-transporter 2 being developed for the treatment of patients with type 2 diabetes mellitus (T2DM). This 52-week study evaluated the efficacy and safety of CANA 300 mg compared with sitagliptin (SITA) in subjects with T2DM inadequately controlled with metformin (MET) plus a sulphonylurea (SU).
Materials and methods: In this randomised, double-blind, active-controlled, Phase 3 study (N = 755), subjects with T2DM inadequately controlled with MET + SU received CANA 300 mg or SITA 100 mg once daily. Changes from baseline in glycaemic and other efficacy endpoints were assessed at Week 52. Adverse events (AEs) were recorded throughout the study.
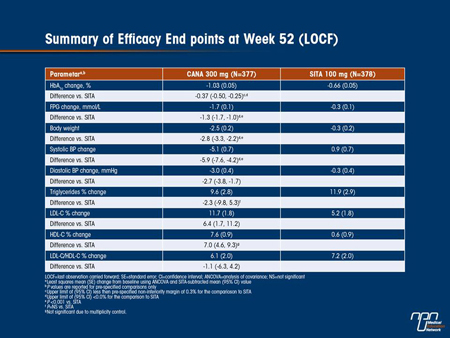
Results: Mean baseline characteristics were similar across groups (age, 56.7 years; HbA1c, 8.1%; body weight, 88.3 kg; body mass index, 31.6 kg/m2). As shown in the Table, HbA1c was reduced from baseline at 52 weeks with CANA 300 mg and SITA 100 mg. CANA 300 mg demonstrated non-inferiority as well as superiority to SITA 100 mg in reducing HbA1c (P <0.001). CANA 300 mg provided consistently lower HbA1c values over 52 weeks while HbA1c increased with SITA 100 mg after Week 12. CANA 300 mg also showed greater reduction in body weight and improvement in fasting plasma glucose (FPG) and systolic blood pressure (BP) compared with SITA 100 mg. CANA 300 mg showed numerical improvement in highdensity lipoprotein cholesterol (HDL-C) and was associated with a slight increase in low-density lipoprotein cholesterol (LDL-C) compared with SITA 100 mg. The overall incidence of AEs was similar with CANA 300 mg (76.7%) and SITA 100 mg (77.5%). Rates of serious AEs (6.4% vs 5.6%) and AE-related discontinuations (5.3% vs 2.9%) were low and similar for CANA 300 mg and SITA 100 mg. Incidences of AEs consistent with superficial genital fungal infections were higher with CANA 300 mg than with SITA 100 mg in women (15.3% vs 4.3%) and men (9.2% vs 0.5%). No differences were observed between CANA 300 mg and SITA 100 mg in the incidences of urinary tract infections (4.0% vs 5.0%) and osmotic diuresis-related AEs
(eg, pollakiuria; <3% per specific AE). The proportion of subjects with ≥1 hypoglycaemia episode were similar with CANA 300 mg (43.2%) and SITA 100 mg (40.7%).
Conclusions: CANA 300 mg showed improvements in glycaemic control, body weight reduction, and systolic BP over 52 weeks compared with SITA 100 mg, and was well tolerated in subjects with T2DM inadequately controlled with MET + SU.
Commentary on abstract 243
This study (DIA3006) showed that in patients with T2DM, canagliflozin given to patients already on metformin plus a sulfonylurea produced significantly improved glycemic control, with greater reductions in BP and weight, compared with established therapy with sitagliptin. However, drop-out rates in the study were high, at 44% of patients on sitagliptin and 33% on canagliflozin, including many
who failed to meet prespecified glycemic targets (23% and 11%, respectively). Some of these patients may have already been getting maximum benefit on the background therapy. Greater increases in LDL-C were seen with canagliflozin, but also in HDL-C and LDL-C:HDL-C. The mechanism of the effect on LDL-C has yet to be identified. More patients on sitagliptin than on canagliflozin were on
statins during and at end of the trial. As expected, the only difference in AEs was the rate of GTIs with canagliflozin, highest in women (15.3%). Incidences of hypoglycemia were similar in both groups, despite better control with canagliflozin.
Questions and answers with Dr. Ronald Goldenberg and Dr. David Lau
Q: Does the 1 year data adequately demonstrate better blood glucose lowering with canagliflozin compared to sitagliptin?
A: Canagliflozin was both non-inferior and superior to sitaglitpin for HbA1c lowering after 1 year of therapy and this is a clinically significant finding.
Q: Is the weight loss over 1 year a benefit for this class of medications?
A: Even a modest weight loss of 2-3% is associated with a multitude of health benefits (glucose, lipids, BP), making this an added benefit of SGLT inhibitors. The interim 1- and 4-year data from the Look AHEAD trial in T2DM suggest that modest weight loss through health behaviour changes is associated with improvement in glycemic, lipid and BP values, and CV disease markers (Wing RR et al. Arch Intern Med 2010;170:1566-75).
Q: If SGLT2 inhibitors are more effective at achieving HbA1c goals compared to DPP-4 inhibitors, where should they be positioned in terms of treatment vs. other antihyperglycemic agents?
A: For patients who would benefit from at least a 0.3 to 0.4% further HbA1c lowering with the added benefit of weight loss and BP lowering, SGLT2 inhibitors may be favoured over DPP-4 inhibitors based on the DIA3006 trial. SGLT2 inhibitors can be used as monotherapy in T2DM in whom metformin cannot be tolerated or contraindicated, as in renal insufficiency with eGFR <60 mL/min. They could also be used in secondline therapy after metformin. Further, because of their unique mechanism of action, SGLT2 inhibitors can also be used to add-on third-line agents to existing antihyperglycemic therapy, including insulin, in patients whose HbA1c were ≤8% but above target levels.
ABSTRACT 763
Efficacy and safety of canagliflozin, a sodium glucose co-transporter 2 inhibitor, compared with glimepiride in patients with type 2 diabetes on background metformin
L. Niskanen, W.T. Cefalu, L. A. Leiter, J. Xie, D. Balis, W Canovatchel, G.Meininger
Background and aims: This 52-week study evaluated the efficacy and safety of canagliflozin (CANA), a sodium glucose co-transporter 2 inhibitor, compared with glimepiride (GLIM) in subjects with type 2 diabetes mellitus (T2DM) inadequately controlled with metformin (MET).
Materials and methods: In this large, randomised, double-blind, active-controlled, Phase 3 study (N=1,450), subjects with T2DM inadequately controlled with MET received CANA 100 or 300 mg or GLIM (doses titrated over 52 weeks up to 6 or 8 mg/day, with mean dose achieved of 5.6 mg). Changes from baseline in efficacy endpoints were assessed at Week 52. Adverse events (AEs) were recorded throughout the study.
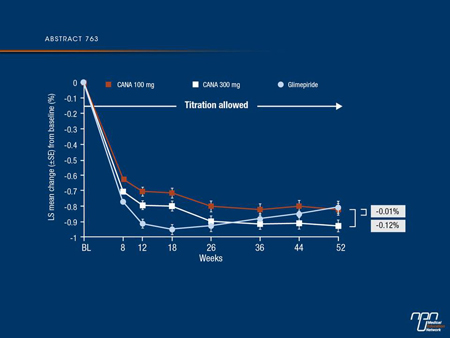
Results: Mean baseline characteristics were similar across groups (age, 56.2 years; HbA1c, 7.8%; body weight, 86.6 kg; body mass index, 31.0 kg/m2). As shown in the Figure, HbA1c (primary endpoint) was reduced from baseline at 52 weeks with CANA 100 and 300 mg and GLIM (least squares [LS] mean changes of -0.82%, -0.93%, and -0.81%). Both CANA doses demonstrated non-inferiority to GLIM in reducing HbA1c, and CANA 300 mg demonstrated superiority to GLIM. CANA 100 and 300 mg provided consistently lower
HbA1c over 52 weeks while GLIM showed increases in HbA1c after Week 18. CANA 100 and 300 mg were superior to GLIM (P <0.001 for all) in body weight reduction (LS mean changes of -4.2, -4.7, and 1.0 kg) and documented hypoglycaemia (defined as ≤3.9 mmol/L) rates (5.6%, 4.9%, and 34.2%). Both CANA doses showed improvements in fasting plasma glucose (FPG), systolic blood pressure (BP), triglycerides, and high-density lipoprotein cholesterol (HDL-C); there was a small dose-related increase in low-density lipoprotein cholesterol (LDL-C) with CANA. More GLIM-treated subjects received rescue therapy (10.6%) than with CANA 100 mg (6.6%) or 300 mg (4.9%). The overall incidence of AEs was similar with CANA 100 mg (64.2%), CANA 300 mg (68.5%), and GLIM (67.6%). Rates of serious AEs (CANA 100 mg, 5.0%; CANA 300 mg, 4.9%; GLIM, 7.9%) and AE-related discontinuations (CANA 100 mg, 5.2%; CANA 300 mg, 6.8%; GLIM, 5.8%) were low and similar across groups. AEs consistent with superficial genital fungal infections were more frequent with CANA 100 and 300 mg than with GLIM in women (14.3% and 23.8% vs 3.7%) and men (6.7% and 8.3% vs 1.1%). CANA showed slightly higher rates relative to GLIM of urinary tract infections (6.4% for both doses vs 4.4%) and osmotic diuresis-related AEs (eg, pollakiuria; <3% for each specific AE).
C o n c l u s i o n s : CANA showed consistent HbA1c lowering over 52 weeks and reduced body weight compared with GLIM, and was well tolerated i n s u b j e c t s w i t h T 2 D M i n a d e q u a t e l y controlled with MET.
Commentary on abstract 763
In this study (DIA3009), canagliflozin compared with the sulfonylurea, glimepiride, in patients with T2DM inadequately controlled on metformin showed consistent HbA1c lowering over 1 year and reduced body weight compared with glimepiride. Both canagliflozin doses showed improvements in FPG, systolic BP, triglycerides and HDL-C; there was a small dose-related increase in LDL-C with canagliflozin. The overall incidence of AEs was similar in all treatment groups. Rates of serious AEs and AE-related discontinuations were low and similar across groups. Consistent with other studies with SGLT2 inhibitors, rates of UTIs, GTIs and osmotic diuresis-related AEs were more frequent with canagliflozin in women and men.
Questions and answers with Dr. Ronald Goldenberg and Dr. David Lau
Q: Is a sulfonylurea the best add-on choice to metformin in terms of durability of blood glucose reduction and safety for patients?
A: A multitude of trials show poor durability with sulfonylurea therapy. While we need longer term trials with SGLT2 inhibitors, this study demonstrated more sustained glucose lowering over 1 year with canagliflozin 300 mg daily compared to glimepiride therapy. Data from the ADOPT study indicated that sulfonylureas were associated with much lower durability than metformin or thiazolidinediones and raised the possibility of causing beta cell exhaustion (Kahn SE et al. N Engl J Med 2006;355:2427-43). While we do not have comparable data on SGLT inhibitors, available data suggest that their efficacy is sustained over a 2-year period. More research trials will clarify the durability of SGLT2 inhibitors.
Q: Should physicians be concerned about the genital infections with canagliflozin?
A: GTIs were generally quite mild, easily treated and rarely led to treatment discontinuation. Furthermore, most patients with these infections usually get a single episode, and recurrent infections were quite rare. SGLT2 inhibitors should be used with caution in women who had known or previous history of recurrent GTIs.
ABSTRACT 721
Sustained reductions in weight and HbA1c with dapagliflozin: long-term results from phase III clinical studies in type 2 diabetes
C.J. Bailey, J. P. H.Wilding, M. Nauck, E. Ferrannini, A.A. Ptaszynska, A.M. Apanovitch, J. Sugg, S.J. Parikh
Background and aims: Dapagliflozin (DAPA), a selective inhibitor of sodium glucose co-transporter 2 (SGLT2), reduces plasma glucose independently of insulin secretion or action, by increasing urinary glucose excretion. In clinical trials up to 2 years, DAPA was associated with a reduction in total body weight (TBW), a key management goal in type 2 diabetes (T2D). This analysis reports TBW and HbA1c from four phase III clinical trials of DAPA, in predominantly overweight/obese patients with T2D, with different treatment
backgrounds, and at different stages of disease.
Materials and methods: The effect of DAPA (10 mg/d) on TBW and HbA1c was assessed in four randomised, double-blind, placebo- (PBO) or comparator-controlled trials: DAPA monotherapy vs PBO (102 wks); DAPA add-on to metformin (MET) [DAPA+MET] vs PBO+MET (102 wks); DAPA+MET vs glipizide (GLIP)+MET (104 wks); DAPA+insulin (INS) [DAPA+INS] vs PBO+INS (104 wks).
Results: In all DAPA groups, the significant reductions in TBW and HbA1c observed at the end of the 24-wk short-term study were sustained for up to 2 years (Figure). At 102/104 wks, DAPA was associated with clinically meaningful reductions in TBW from baseline (BL) in each study (range: -1.74 to -4.05 kg). In contrast, over the same time period, weight gain from BL was observed in the PBO+MET (+1.36 kg), GLIP+MET (+1.36 kg) and PBO+INS (+0.91 kg) groups. PBO- or comparator-corrected adjusted mean reductions in TBW were in the range -2.41 to -5.06 kg. In addition, DAPA produced clinically meaningful reductions in HbA1c from BL (range: -0.32 to -0.78%), with PBO- or comparator-corrected adjusted mean reductions in HbA1c in the range -0.18 to -0.80%. In the DAPA groups, systolic blood pressure was either reduced or maintained in all studies (mean change from BL; range: -0.3 to -5.9 mmHg). Urinary glucose/creatinine ratio was elevated and maintained in all studies, supporting the long-term efficacy of DAPA as an SGLT2 inhibitor. Adverse events were generally balanced across the groups. DAPA (10 mg/d) was not associated with a higher risk of hypoglycaemia. Events suggestive of genital and urinary tract infection were greater with DAPA. Infections occurred more often in the first 24 wks, were mostly mild to moderate in severity, managed by standard interventions, and without serious clinical consequences.
Conclusions: In populations of overweight/obese patients with T2D, treated across the typical course of the disease, DAPA administered as monotherapy or in combination with MET or INS was associated with sustained decreases in both TBW and HbA1c, over 2 years.
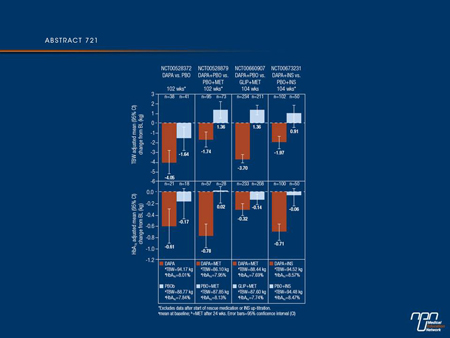
Commentary on abstract 721
This analysis of pooled data from 4 phase III trials has shown that in overweight/obese (BMI ≥25) diabetic patients the use of an SGLT2 inhibitor, in this case, dapagliflozin, is able to increase glucosuria, which is maintained independently of insulin over 2 years, and is able to produce consistent reductions in weight at the same time as reductions in HbA1c. This effect was observed whether dapagliflozin was administered as monotherapy or as add-on to metformin or insulin and whether patients received their diabetes diagnosis within 2 to 14 years. In this analysis, GTIs and UTIs were more frequent with the SGLT2 inhibitor than either placebo or glipizide, the active comparator in one of the trials. They occurred more often in the first 24 weeks, were mostly mild to moderate in severity, and were managed by standard interventions.
Questions and answers with Dr. Ronald Goldenberg and Dr. David Lau
Q: Does weight loss with dapagliflozin occur only in overweight patients?
A: In a pooled analysis of dapagliflozin trials, weight loss was independent of baseline BMI. Because of its glucosuric effect, SGLT2 inhibitor therapy would lead to modest weight loss regardless of the baseline body weight of patients with T2DM.
Q: Is hypoglycemia less common with dapagliflozin than other antihyperglycemic agents?
A: SGLT2 inhibitors are not associated with an increased risk of hypoglycemia when given as monotherapy or in combination with other agents that do not increase the risk of hypoglycemia. However, hypoglycemia rates can increase when SGLT2 inhibitors are added to such agents as sulfonylureas or insulin, which in turn may necessitate a reduced dosage of the sulfonylurea or insulin. SGLT2 inhibitors are true antihyperglycemic agents and, as such, hypoglycemia is only seen when used in combination therapy with other glucose-lowering agents, especially insulin secretagogues and insulin, in which case dosage reduction of the latter might be necessary.
ABSTRACT 762
Canagliflozin, a sodium glucose co-transporter 2 inhibitor, reduces body weight mainly through loss of fat mass in subjects with type 2 diabetes
S. Toubro, W.T. Cefalu, J. Xie, D. Sullivan, K. Usiskin, W. Canovatchel, G. Meininger
Background and aims: Canagliflozin (CANA) is a sodium glucose co-transporter 2 inhibitor that improves the glycaemic profile and reduces body weight in type 2 diabetes mellitus (T2DM) subjects. Body composition analysis was conducted to determine the relative contributions of weight loss from fat and lean mass in a subset of subjects with T2DM enrolled in 2 randomised, double-blind, Phase 3 studies.
Materials and methods: Study 1 was an active-controlled trial comparing CANA 100 and 300 mg with glimepiride (GLIM) in T2DM subjects inadequately controlled on metformin (N = 1,450; mean baseline characteristics: age, 56.2 years; HbA1c, 7.8%; body weight, 86.6 kg). Study 2 was a placebo (PBO)-controlled trial that evaluated CANA 100 and 300 mg in subjects aged 55 to 80 years (N = 714) with T2DM on a variety of background anti-hyperglycaemic agents (AHAs); mean baseline characteristics were similar across groups (age, 63.6 years; HbA1c, 7.7%; body weight, 89.5 kg). Body composition was assessed in a subset of subjects by dual-energy X-ray absorptiometry (DXA) at 52 weeks in Study 1 (n = 208) and at 26 weeks in Study 2 (n = 211). Abdominal fat distribution was also assessed by computed tomography (CT) scans at 52 weeks in Study 1 only (n = 217).
Results: In T2DM subjects on background metformin (Study 1 [Table]), CANA 100 and 300 mg significantly reduced body weight compared with GLIM (P <0.001 for both) at 52 weeks (least squares [LS] mean changes from baseline of -4.2, -4.7, and 1.0 kg, respectively). Loss of fat mass accounted for approximately two-thirds of the overall body weight reduction seen with CANA. Both CANA doses also showed reductions in percent total fat (fat percentage of the total weight) compared with GLIM. Similar results were observed in Study 2 subjects on a variety of background AHAs (Table). CANA 100 and 300 mg significantly reduced body weight compared with PBO (P <0.001 for both) after 26 weeks of treatment (LS mean changes from baseline of -2.5, -3.3, and -0.2 kg, respectively). Greater loss from fat mass than from lean mass was observed for CANA 100 and 300 mg, and both CANA doses showed reductions compared with PBO in percent total fat. Analysis of abdominal fat in Study 1 showed slightly greater changes in the amount of visceral adipose tissue (LS mean changes relative to GLIM of -7.4% and -8.3%, respectively) than changes in subcutaneous adipose tissue (LS mean changes relative to GLIM of -7.2% and -7.4%, respectively) with CANA 100 and 300 mg.
Conclusions: The body weight reductions seen in subjects with T2DM following 26 or 52 weeks of treatment with CANA 100 or 300 mg were predominantly (>2/3) from fat mass. There was a numerically greater percentage loss of visceral relative to subcutaneous fat in the abdomen.
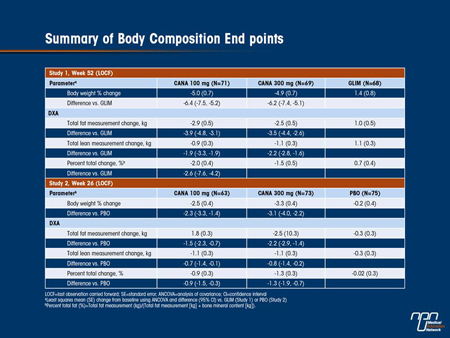
Commentary on abstract 762
Body composition analysis has shown that the weight loss produced by canagliflozin at doses of 100 and 300 mg/day in patients with diabetes is mainly due to loss of fat mass. This was studied in subjects taking part in a trial of canagliflozin vs. glimepiride (which is associated with weight gain) as add-on therapy to metformin and in patients in another trial who were given canagliflozin in addition to a variety of antihyperglycemic agents. Slightly greater fat loss came from visceral fat than from subcutaneous fat, according to computed tomography (CT) scans. These reductions in fat relative to lean mass are consistent with similar findings with other antihyperglycemic drugs that induce weight loss, including studies with other SGLT2 inhibitors presented at the meeting.
Questions and answers with Dr. Ronald Goldenberg and Dr. David Lau
Q: What is the significance of reduction in visceral vs. subcutaneous adipose tissue?
A: Any fat loss is beneficial in improving cardiometabolic risk. However, loss of intra-abdominal or visceral vs. subcutaneous fat is associated with greater improvement in obesity-related metabolic complications, such as insulin resistance, atherogenic dyslipidemia, hypertension and hypercoagulable states.
Q: Should one recommend an exercise program at the same time for greater weight loss?
A: Exercise and regular physical activity should be recommended for all people and especially in T2DM because exercise can reduce insulin resistance, improve glycemic and metabolic control, and CV health.
Q: Should reduction in total body weight be a goal for all classes of antihyperglycemic medicine?
A: Even a modest weight gain can be associated with worsening in glycemic and metabolic control, and CV risks. Hence avoidance of weight gain should be an integral consideration in choosing glucoselowering therapy for T2DM. Data from the DCCT-EDIT follow-up trial in T1DM suggested that even a modest weight gain of a few kg from insulin therapy could lead to worsening in lipid profiles and CV risk factors (N Engl J Med 2005;353:2643-53).
Q: Can a synergistic effect on BP with canagliflozin be expected and should clinicians monitor BP?
A: SGLT2 inhibitors are associated with modest reduction in BP attributable at least partly to its diuretic effect. While regular BP monitoring is not required, orthostatic hypotension could occur in a small number of patients, especially those who are on antihypertensive therapy. Clinicians should be alerted to the BP-lowering properties of SGLT2 inhibitors.
ABSTRACT 690
Weight gain within the first year after new onset diabetes is associated with risk of cardiovascular mortality: a cohort of 8,326 primary care patients
J. Bodegard, J. Sundström, B. Svennblad, C. Östgren, P.M. Nilsson, G. Johansson
Background and aims: Increased body mass index (BMI) in diabetes patients has been suggested to be associated with higher coronary heart disease risk. We explored the association between BMI changes within the first year of new onset diabetes (NOD) and the risk of cardiovascular disease (CVD) mortality.
Materials and methods: A cohort of 38,960 diabetes patients with NOD, no history of previous CVD or cancer, and available BMI measurements was identified at 84 primary care centers in Sweden (1999 - 2009). Of these; 8,326 patients had their BMI measured at least twice, at the time of NOD and within one year after the NOD diagnosis. After the last BMI measurement, the patients were followed for up to 10 years. Morbidity- and mortality data was retrieved by linking these patients to National Cause of Death and Hospital Discharge registers. Patients were grouped according to one year BMI change after NOD: “Increase” ≥1 BMI unit increase; “Unchanged” = between +1 and -1 BMI unit change; “Decrease” ≥1 BMI unit decrease. CVD risks were estimated with Cox regression, adjusted for age, gender, baseline BMI, history of angina pectoris, education, marital status and glucose lowering drugs. CVD mortality includes
sudden and unexpected death, death from stroke and myocardial infarction.
Results: Baseline mean age was 60.0 years (range 35-79) and mean BMI 30.2 kg/m2 (range 16.7-58.5). After a median follow up time of 4.6 years (38,300 patient years), most patients, 53.4%, had an unchanged BMI, 32.2% a decrease, and 14.4% an increase. At baseline, patients with increasing BMI were younger, had lower HbA1c, triglycerides and systolic blood pressure, and were during the first year with NOD more frequently on insulin and sulfonylureas; less frequently on metformin and antihypertensives compared to
the unchanged group.Patients in the increased BMI group had a 63% (95%CI 1.11-2.39) increased risk of CVD death compared to patients with unchanged BMI, see figure, and all cause death increased by 33% (95%CI 1.01-1.76).In a subgroup analysis of the 3,034 patients (36.4%) increasing their BMI >0 in the first year with NOD; 100 (3.3%) died from CVD and every 1 unit BMI increase was associated with 12% increased risk of CVD death (95%CI 0.94-1.33).
Conclusions: Increase in body mass index during the first year after new onset diabetes is associated with increased long term risk of death due to cardiovascular disease. The study also suggests that the level of BMI increase is associated with an elevated risk of dying from cardiovascular disease and every effort should be made to minimize increase in BMI in diabetes patients.
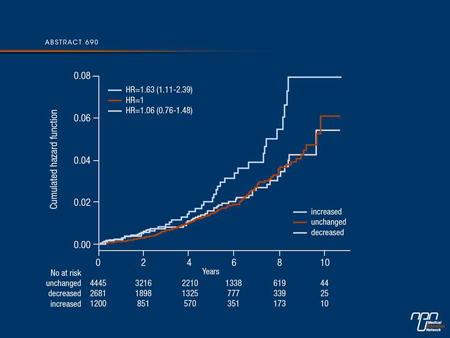
Commentary on abstract 690
People with T2DM are known to have increased CV risk, and such risk appears to be exaggerated in those with higher BMI values. This study explored the association between BMI changes within the first year of new onset diabetes (NOD) and the risk of mortality in over 8000 primary care patients (average age 60 years) identified from Swedish national registries. They were diagnosed with NOD between 1999 and 2008 and they had their BMI measured at the time of diagnosis and 1 year later, with no change in 53.4%, a decrease in 32.2% and an increase in 14.4%. After an average follow-up of almost 5 years, the increased BMI group was found to have increased risks of CV and all-cause mortality. A surprising finding was that this group was more highly educated than the other groups. It is notable that at least during the earlier years of patient diagnosis, patients in this group were more likely to be prescribed insulin or a sulfonylurea drug, possibly leading to more hypoglycemic episodes and partly explaining the increased risk.
Questions and answers with Dr. Ronald Goldenberg and Dr. David Lau
Q: Can a reduction in CV risk be expected with antihyperglycemic agents that also reduce body weight?
A: The Look AHEAD Trial is the only study to date testing the hypothesis of reducing CV outcomes in people with T2DM by health behaviour changes. Unfortunately the study was terminated prematurely because of the lack of CV benefit between the intensively treated and conventional treatment groups. This is partly due to the much lower than expected CV events that occurred in the study subjects, and that the statin-treated LDL-C levels of the subjects in the conventional arm were lower than those of the intensively treated groups. While awaiting the published results of the Look AHEAD trial, whether weight loss associated with glucoselowering agents would confer CV benefits in T2DM remains speculative. Nonetheless, modest weight loss confers other health benefits and remains a cornerstone therapy in T2DM.
Q: Since an increase in BMI in new onset diabetes is associated with an increase in CV risk, what preventative measures might be recommended?
A: It is evident that weight loss is beneficial in improving the health status of people with T2DM, health behaviour changes are recommended. Achieving a healthier body weight through proper eating habits and regular physical activity should be a goal not only to prevent or delay T2DM, but also for its ongoing management.
ABSTRACT 759
Canagliflozin, a sodium glucose co-transporter 2 inhibitor, improves glycaemic control and is well tolerated in type 2 diabetes subjects with moderate renal impairment
J.-F. Yale, G. Bakris, E. Wajs, L. Xi, K. Figueroa, K. Usiskin, G. Meininger
Background and aims: Canagliflozin (CANA), a sodium glucose co-transporter 2 inhibitor, lowers plasma glucose by increasing urinary glucose excretion. This randomised, double-blind, placebo (PBO)-controlled, Phase 3 study evaluated the efficacy and safety of CANA in subjects with type 2 diabetes mellitus (T2DM) and moderate renal impairment.
Materials and methods: Subjects with T2DM (N = 269) who had moderate renal impairment (estimated glomerular filtration rate [eGFR]) ≥30 and <50 mL/min/1.73 m2) received CANA 100 or 300 mg or PBO once daily. Efficacy endpoints were assessed at Week 26 and adverse events (AEs) were recorded throughout the study. Renal safety parameters were also evaluated.
Results: Mean baseline characteristics were similar across groups (age, 68.5 years; HbA1c, 8.0%; body weight, 91.2 kg; body mass index, 33.0 kg/m2; eGFR, 39.4 mL/min/1.73 m2); median baseline albumin/creatinine ratio (ACR) was 30.0 μg/mg. 74.0% of subjects were on insulin and 31.2% were on a sulphonylurea. As shown in the Table, HbA1c was significantly decreased at Week 26 with CANA 100 and 300 mg compared with PBO (P<0.05 for both). Both CANA doses showed numerical improvements in fasting plasma glucose
(FPG) and reductions in body weight and systolic blood pressure (BP) compared with PBO. More PBO-treated subjects received rescue therapy (14.4%) than with CANA 100 or 300 mg (4.4% and 3.4%, respectively). The overall incidence of AEs was slightly higher for CANA 100 and 300 mg than for PBO (77.8% and 74.2% vs 73.3%); serious AE rates were 11.1%, 11.2%, and 17.8%, respectively. AE-related discontinuation rates were low across groups. Incidences of superficial genital fungal infections were higher with CANA 100 and 300 mg than PBO (women, 6.3% and 4.9% vs 0%; men, 1.7% and 2.1% vs 0%). CANA 300 mg showed slightly higher urinary tract infection rates (7.9%) than other groups (5.6% for CANA 100 mg and PBO); no increases in upper tract infections were seen with CANA. Incidences of osmotic diuresis- and volume-related AEs were higher with CANA than with PBO (<5% per specific AE except hypotension [6.7%] and dizziness [5.6%] for CANA 300 mg). More subjects had ≥1 hypoglycaemia episode with CANA 100 and 300 mg than PBO (52.9% and 51.2% vs 36.4%). CANA 100 and 300 mg showed increases in serum creatinine (9% and 10% vs 4%) and blood urea nitrogen (9% and 6% vs 2%) compared with PBO. Changes in eGFR for CANA and PBO were approximately -10% (both doses) and -3%, respectively. Median ACR was reduced with CANA 100 and 300 mg compared with PBO (-21.6% and -12.4% vs -2.5%).
Conclusion: CANA was well tolerated and demonstrated efficacy in improving glycaemic control and reducing body weight in subjects with T2DM who had moderate renal impairment.
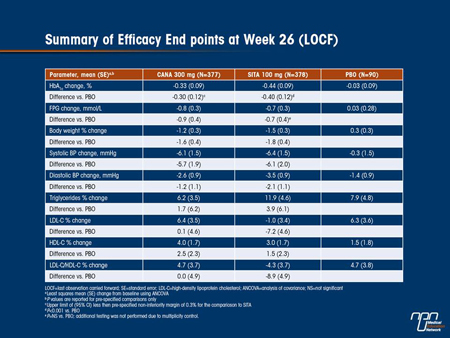
Commentary on abstract 759
Renal safety of SGLT2 inhibitors is of special interest, given their mechanism of action targeting renal action and they might be expected to have reduced efficacy in patients with diabetes and renal impairment. In this study, canagliflozin significantly reduced HbA1c at week 26 vs. placebo, and although the reduction was not as great as seen in patients with normal renal function, it was deemed clinically significant. Similar reduction differences were seen in fasting plasma glucose, body weight and SBP. Risk of hypoglycemia was increased in patients on background insulin or insulin secretagogues, but not in patients not on these therapies. Small increases were observed in AST, ALT, Hb and serum magnesium. Some changes occurred in renal parameters, such as decreased eGFR and urine albumin:creatinine ratio and increased BUN. Slightly higher rates of UTIs and adverse events related to osmotic diuresis and reduced intravascular volume were reported with the higher canagliflozin dose. Changes in lipids were consistent with other studies, except for
a decrease in LDL-C seen only with the higher canagliflozin dose.
Questions and answers with Dr. Ronald Goldenberg and Dr. David Lau
Q: Do patients with T2DM in clinical practice have decreased eGFRs and does canagliflozin provide efficacy in patients with reduced eGFR?
A: About 40% of patients with T2DM have renal complications and approximately 20% have eGFR levels below 60 ml/min. Depending on the studies, the prevalence of chronic kidney disease (CKD) is high among T2DM and much higher than recognized. Glycemic management of people with T2DM and CKD with decreased eGFR is often challenging.
Q: What does this data mean for physicians and patients clinically?
A: Clinicians must be aware of the efficacy and safety of antihyperglycemic agents in patients with CKD, and know the appropriate doses in this population. In CKD patients where metformin therapy is contraindicated in patients with eGFR <30 mL/min, repaglinide,
insulin and more recently DPP-4 inhibitors are the only available antihyperglycemic agents. SGLTs inhibitors retain their efficacy albeit less in patients with mild to moderate renal impairment (eGFR <60ml/min) and could theoretically be used as an alternative.
Q: Which dose should be recommended for patients with mild renal insufficiency?
A: Patients with mild CKD can be treated with the usual doses of canagliflozin. For those with moderate CKD, 100 mg may be the safest starting dose, as there were less volume related adverse events compared to 300 mg in this patient population.
Q: Does this suggest that all SGLT inhibitors can be given to patients with moderate renal impairment?
A: SGLT2 inhibitors become less efficacious as renal function declines. This study demonstrates modestly effective glucose lowering with canagliflozin in patients with moderate renal impairment. In a study with dapagliflozin in patients with moderate renal impairment (GFR 30-59 ml/min), dapagliflozin did not improve HbA1c, yet a pooled analysis of dapagliflozin 10 mg in patients with GFR in the 45 to 59 ml/min range did demonstrate a modest lowering of HbA1c. Individual SGLT2 inhibitors may differ in their efficacy in patients with mild to moderate renal impairment. Further research will establish the role of SGLT2 inhibitors in moderate renal impairment, but because of the mode of action, this class of glucose-lowring agents is likely to be ineffective in severe renal impairment.
ABSTRACT 744
Dapagliflozin improves glycaemic control and reduces body weight across various patient populations with type 2 diabetes mellitus
S. Parikh, E. Hardy, L. Wei, C. Wessman, A. Salsali, A. Ptaszynska
Background and aims: Dapagliflozin (DAPA), a selective sodium-glucose cotransporter 2 (SGLT2) inhibitor, reduces plasma glucose independently of insulin by increasing renal glucose excretion. The efficacy of DAPA in patients with T2DM across 5 clinical trials was assessed.
Materials and methods: Patients with T2DM received DAPA (2.5, 5, or 10 mg/d) or placebo for 24 wk as monotherapy (N=274, main cohort) or add-on to metformin (MET, N=546), glimepiride (GLIM, N=596), pioglitazone (PIO, N=420) or insulin (INS, N=807). The primary endpoint for all trials was mean change from baseline in HbA1c at week 24. Secondary endpoints included change from baseline in fasting plasma glucose (FPG), postprandial glucose (PPG, tested in add-on to GLIM and add-on to PIO studies), and body weight. The primary endpoint was analyzed by ANCOVA, excluding data after rescue (last observation carried forward). Secondary endpoints were analyzed by hierarchical testing.
Results: Mean baseline HbA1c ranged from 7.9% to 8.5%. Placebo-corrected adjusted mean changes from baseline in HbA1c for DAPA 10 mg/d ranged from -0.5% to -0.7% (P<0.001 in all studies) (Figure). DAPA also reduced FPG in all studies, and PPG in the 2 trials where it was tested. Body weight was reduced by 1.5 to 2 kg with DAPA 10 mg/d compared with placebo in the add-on to MET, add-on to GLIM, add-on to PIO, and add-on to insulin trials (P<0.0001 in all 4 studies). Placebo-corrected weight reductions of up to 1 kg were observed in the monotherapy trial, however these changes did not reach statistical significance due to a greater-thanexpected weight effect in the placebo group. Dapagliflozin was generally safe and well tolerated in these trials. Signs and symptoms suggestive of urinary tract infection and genital infection were more commonly reported as adverse events in the dapagliflozin dose groups compared with placebo.
Conclusions: DAPA produced significant improvements in glycaemic control and body weight in various patient populations with T2DM across the treatment continuum, consistent with its insulin-independent mechanism of action.
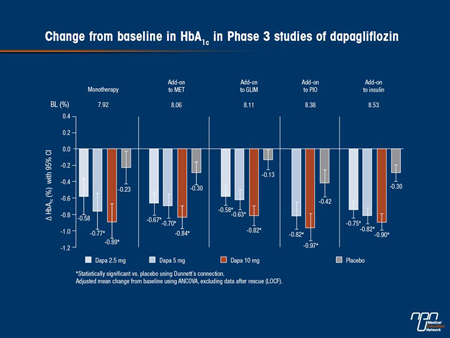
Commentary on abstract 744
The patients in these clinical trials had improved glycemic control and reduced body weight compared with placebo regardless of disease severity or duration when used as monotherapy or as add-on to 4 commonly used drugs with different modes of action (metformin, glimepiride, pioglitazone or insulin). Weight loss was seen with dapagliflozin in all studies except in combination with pioglitazone where weight remained unchanged, suggesting that dapagliflozin mitigated the pioglitazone-induced weight gain. Rates of hypoglycemic episodes were low with SGLT2 inhibitor monotherapy or in combination with metformin or pioglitazone, but higher in combination with glimepiride or insulin, as previously observed when agents with low hypoglycemic propensity are added to a sulfonylurea or insulin. As in other studies, compared with placebo, higher rates of events suggestive of GTIs were reported in patients taking dapagliflozin.
Questions and answers with Dr. Ronald Goldenberg and Dr. David Lau
Q: What is the clinical significance of the insulin-independent mechanism of action of antihyperglycemic medications?
A: An insulin-independent mechanism of action means that a class of agent is expected to be effective no matter the degree of beta-cell function or insulin resistance in patients with T2DM.
Q: Which classes could be considered to have an insulin-independent mechanism of action?
A: Alpha-glucosidase inhibitors have an insulin-dependent mechanism of action by reducing postprandial hyperglycemia. SGLT2 inhibitors are also insulin-independent as they exert their glucose-lowering effects by reducing glucose reabsorption in the proximal renal tubules.
ABSTRACT 743
Safety of dapagliflozin in clinical trials for type 2 diabetes mellitus
K. Johnsson, A. Ptaszynska, A. Apanovitch, J.E. Sugg, S.J. Parikh, J.F. List
Background and aims: Dapagliflozin (DAPA), a sodium glucose cotransporter 2 (SGLT2) inhibitor, reduces hyperglycaemia in an insulin-independent manner by increasing renal glucose excretion, and represents a potential option for T2DM patients. The aim of this analysis was to assess safety across the DAPA clinical development programme.
Materials and methods: Data were pooled from 12 phase 2b/3 short-term double-blind placebo (PBO)-controlled trials (4684 patients) to examine overall safety. An updated database of 19 trials (8685 patients; 1.8:1 cumulative exposure ratio for DAPA:control) was used to examine rare events.
Results: Adverse events (AEs) were slightly more common with DAPA vs PBO; serious AEs and discontinuations due to AEs were balanced across groups (Table 1). Hypoglycaemia was more common with DAPA vs PBO, driven by studies in which DAPA was combined with sulphonylurea or insulin. AEs of genital infections and UTI were more common with DAPA vs PBO with imbalances less marked for UTIs. These were mild/moderate, responded to standard antimicrobial therapy and rarely led to discontinuation. Mean changes from baseline in systolic/diastolic BP were -4.0/-2.0 vs -0.9/-0.5 mmHg for DAPA vs PBO without increases in measured
orthostatic hypotension (3.9% vs 3.7% for DAPA vs PBO). AEs of volume depletion (hypotension/dehydration/hypovolaemia) were seen in 0.6-1.2% for DAPA 0.4% for PBO. AEs of renal impairment/failure were balanced across groups (0.9-1.4% for DAPA vs 0.9% for PBO). Minor changes in serum electrolytes were seen with DAPA, with small increases in serum Mg and P. PBO-corrected reductions in uric acid, in the studies it was analysed, ranged from -0.45 to -0.8 mg/dL (but reductions of less magnitude were noted in an add-on to
insulin and a phase 2b monotherapy study). Incidence rates/100 pt years of malignant and unspecified tumours were similar for DAPA (1.39) vs control (1.34). Incidence rates of bladder cancer were 0.15 for DAPA vs 0.03 for control with an incidence rate (IR) difference of 0.117 (95%CI: -0.171, 0.367) and of breast cancer were 0.37 vs 0.22 respectively with an IR difference of 0.228 (95%CI: -0.537%, 0.806). Haematuria was noted at or before baseline in 7/10 patients with bladder cancer. All breast cancer events were reported within the first year of study initiation. Non-clinical DAPA data did not indicate any carcinogenic potential, and SGLT2 is selectively expressed in the kidney with no expression in human breast or bladder tissue. Elevated liver laboratory tests were seen in 4.4% vs 4.2% for DAPA vs control. Hazard ratio for CV death, MI, stroke or hospitalization for unstable angina for DAPA vs control was 0.82 (95% CI: 0.583, 1.152). Trends in CV events and specific malignancies will be further evaluated in a randomised CV outcomes trial and complementary observational studies.
Conclusions: Based on this large phase 2b/3 experience with >4500 T2DM patients, DAPA has an acceptable safety profile.
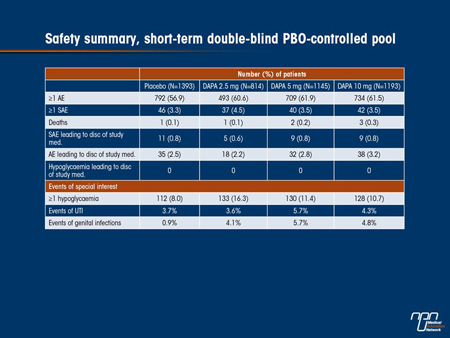
Commentary on abstract 743
The dapagliflozin clinical development program has data for up to 2 years in over 8000 patients showing that it is safe and well tolerated in patients across the diabetes spectrum. Rates of GTIs and UTIs were slightly higher with dapagliflozin, but they were mild to moderate, occurred in the first 6 months of treatment, responded to standard antimicrobial therapy and seldom led to discontinuation of study treatment. There was no increased risk of hypoglycemia, renal impairment or elevated liver function tests, or unacceptably increased risk of CV outcomes. Incidences of malignant and unspecified tumours were similar for dapagliflozin (1.47%) vs. control (1.35%), although higher for bladder cancer (0.16% vs. 0.03%) and for prostate and breast cancers (0.18 vs. 0.09 for both), possibly chance
findings. Most patients with bladder cancer had hematuria at baseline, indicating that some of the tumours might have been pre-existing. The imbalance for breast cancer decreased over time, as would be expected if this were a random finding. Furthermore, most cases of bladder cancer and all cases of breast cancer occurred within one year of study start, making a drug induced cancer very unlikely. SGLT2 is not expressed in human breast or bladder tissue, so we would not expect SGLT2 inhibition to have an impact in these tissues.
Questions and answers with Dr. Ronald Goldenberg and Dr. David Lau
Q: Are volume depletion adverse events of particular concern to the practicing physician?
A: Adverse events of volume depletion, such as hypotension or dehydration were rare and of little concern. However, there is a slight increase in these events in patients at risk of volume depletion, such as those on loop diuretics. For these patients a lower starting dose may be beneficial.
Q: Are there any adverse event in this analysis that should receive special attention?
A: Further studies and ongoing follow-up of dapagliflozin-treated patients will help to establish if the increased rate of bladder and breast cancer was a chance finding.