Reports
Young Infants and the Multi-Component Serogroup B Vaccine: New NACI Recommendations for Healthcare Professionals
This report is based on medical evidence presented at sanctioned medical congress, from peer reviewed literature or opinion provided by a qualified healthcare practitioner. The consumption of the information contained within this report is intended for qualified Canadian healthcare practitioners only.
MEDI-NEWS - Based on Guidelines Prepared by the Meningococcal B Pilot Project Task Group
March 26, 2014
Invasive meningococcal disease (IMD) is an acute and serious infection caused by the bacterium Neisseria meningitidis. In Canada, 5 serogroups are responsible for IMD, of which serogroup B is now the most prevalent, especially among infants under one year of age; children 1 to 4 years of age, and adolescents 15 to 19 years of age. Based on their review of an extensive development program, the National Advisory Committee on Immunization (NACI) now recommends that healthcare professionals consider using the new multi-component vaccine in infants 2 months of age and older to protect them against IMD caused by serogroup B. Individuals who are at high risk for or who have been exposed to any person with serogroup B IMD should also receive the vaccine. When given concomitantly with routine infant vaccines, prophylactic acetaminophen may be administered with the vaccine to reduce fever risk. Alternatively, the vaccine may be given separately from routine infant vaccinations to minimize fever as well.
Chief Medical Editor: Dr. Léna Coïc, Montréal, Quebec
The new multi-component vaccine against serogroup B Neisseria meningitidis (4CMenB or Bexsero ) may be considered in infants 2 months of age and older to prevent invasive meningococcal disease (IMD) caused by serogroup B bacterium.
And in individuals 2 months of age and older at high risk for, or who have been in close contact with any person who developed IMD caused by serogroup B, the same vaccine should also be given. As the Meningococcal B Pilot Project Task Group points out, serogroup B is responsible for most IMD in Canada, although incidence varies by age, geographic area and time of year.
Introduction of the meningococcal C conjugate vaccine into routine immunization programs in recent years has meant that serogroup B now accounts for the greatest proportion of reported IMD cases in Canada—62% in 2011 compared with only 2% due to serogroup C. Between 2007 and 2011, an average of 192 cases of IMD a year were reported in Canada—an average of 111 of them due to serogroup B.
The incidence of serogroup B disease also remains highest in infants under the age of 12 months, at an age-specific incidence rate of 5.8 cases per 100,000 in 2011. This is followed by incidence rates of 1.4 cases per 100,000 in infants between the ages of 1 to 4 years and an incidence rate of 0.7 out of 100,000 among 15 to 19-year olds. The incidence of serogroup B among 15 to 19-year olds has been particularly high in Quebec relative to other regions, Task Group members add.
4CMenB Efficacy
As the Task Group explains, the 4CMenB vaccine is the first vaccine to have been created through a process known as “reverse vaccinology”. “Through this process, potential vaccine targets (i.e., antigens) are identified and developed by sequencing the meningococcal serogroup B genome,” they write. Specifically, the vaccine contains 3 recombinant proteins combined with outer membrane vesicles derived from an epidemic meningococcal strain.
This means that the vaccine is protective only against strains that express antigens contained in the vaccine, as Task Group members point out. That said, a significant proportion of serogroup B strains do express vaccine-containing antigens. Furthermore, antigens contained in the vaccine are not entirely unique to serogroup B and may be expressed by other meningococcal serogroups, the authors add.
A total of 10 clinical trials involving approximately 5800 healthy subjects—some 4000 of whom were infants between the ages of 2 and 24 months—have evaluated the immunogenicity of the new serogroup B vaccine. Each trial assessed immune responses to each vaccine antigen independently using a human complement serum bactericidal assay (SBA) titre against reference strains. Reference strains evaluated in these studies included factor H binding protein (fHbp); Neisserial adhesion A (NadA), and outer membrane vesicles from a N. meningitidis group B strain NZ98/254 (PorA). It is important to note that studies were conducted prior to the identification of a reference strain that primarily expresses the Neisseria Heparin Binding antigen (NHBA) which is also contained in the vaccine.
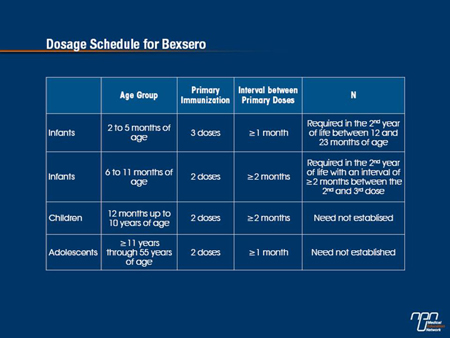
“In infants aged ≤12 months, 4CMenB vaccine was found to be immunogenic after at least 2 doses and an anamnestic response to a booster dose, given at 12 months of age, was also evident,” the Task Group observes. A variety of vaccine schedules were assessed, they add, with no one schedule resulting in superior immunogenic responses than another.
Immune responses to the 4CMenB vaccine were also similar when the vaccine was given together with other routine childhood vaccines or administered separately, they add. The same vaccine was found to be immunogenic against reference strains after 2 doses in older children (12 to 24 months). A small number of children also received 2 doses of the vaccine at 40 and 42 months of life. Again, seroprotective antibody titres were achieved after the second dose for each of the reference strains in the majority of participants.
Trials of the 4CMenB vaccine in both adolescents and adults indicate that most adolescents achieved protective antibody responses against each of the 3 reference strains after 2 to 3 doses as did adults on receipt of 2 doses.
In Canada, the 4CMenB vaccine has been authorized for use in recipients from the age of 2 months through to the age of 17.
It is noteworthy, however, that data reported in the 4CMenB clinical trials program indicates the vaccine is safe and immunogenic in adults up to 55 years of age using the 2-dose schedule with an interval of at least 1 month between doses.
Safety Profile
Nine out of 10 clinical trials also reported safety outcomes. From these studies, the most commonly reported reactions following vaccination in infants up to the age of 12 months were erythema, induration, fever and sleepiness or irritability. Similar proportions of local reactions to the 4CMenB vaccine were reported when the vaccine was given either together with routine infant vaccines or separately, although the incidence of pain was higher following concomitant administration, as was the incidence of fever.
In children two yrs of age and under, fever was more common after the first or second dose of 4CMenB vaccine than after the third dose and occurred mostly within the first six hours after vaccine administration,Task Group members note. The same sort of local reactions were observed in infants between 12 and 24 months of age and more children in the same age group experienced fever when the 4CMenB vaccine was given together with a few other routine childhood vaccines.
Importantly, acetaminophen has been found to significantly lower the incidence of febrile events following 4CMenB vaccination without affecting the immunogenicity of either 4CMenB or any routine vaccines given concomitantly with it. These findings suggest that routine prophylactic administration of acetaminophen may be an appropriate strategy to counter fever among infants receiving the 4CMenB vaccine, as Task Group members suggest.
Alternatively, physicians may consider separating the 4CMenB vaccine from routine infant vaccination schedules to prevent fever in infants up to 3 years of age. When giving the vaccine, parents should also be counseled about the potential for infants to develop a local or systemic reaction and be instructed on appropriate management strategies.
4CMenB in Canada
Debate continues on how protective the new multi-component serogroup B vaccine will be in the Canadian context. Based on the MATS assay, IMPACT (Immunization Monitoring Program Active) study investigators estimated that approximately two-thirds of the overall proportion of Canadian serogroup B strains should be susceptible to the 4CMenB vaccine. At the same time, the Task Group emphasizes that there are areas of uncertainty concerning the vaccine’s coverage of circulating serogroup B strains in Canada as well as questions about its effectiveness and adverse effects at the population level.
Nevertheless, IMD is associated with a high burden of disease. In an IMPACT study of 413 confirmed Canadian serogroup B cases hospitalized between 2002 and 2011, mean length of hospital stay was 11.2 days and some 60% of cases required ICU care. Among patients admitted to the ICU, 45% required assisted ventilation. Some 319 patients survived and of the survivors, 19% had at least one sequelae due to the infection and 23% required inpatient rehabilitation. The most commonly reported sequelae included deafness, skin scarring, amputation, neurologic adverse outcomes, seizures and renal dysfunction.
Clearly, IMD, though rare, is a catastrophic disease and one well worth preventing regardless of the serogroup from which it arises.
MEDIMN