Reports
A deep dive into neurofibromatosis type 1: Expert experiences and perspectives from SNO 2023
This report is based on medical evidence presented at sanctioned medical congress, from peer reviewed literature or opinion provided by a qualified healthcare practitioner. The consumption of the information contained within this report is intended for qualified Canadian healthcare practitioners only.
PRIORITY PRESS - SNO 2023: Annual Meeting of the Society for NeuroOncology
Vancouver, British Columbia / November 15–19, 2023
Vancouver – The Society for NeuroOncology (SNO) is an international organization that brings together clinicians and researchers with an interest in central nervous system malignancies. Although a large part of its focus is on diagnosis and management of malignant brain tumours, this year’s SNO conference provided valuable educational and scientific sessions on other malignant and non-malignant tumours affecting nerves and supporting cells throughout the body. Of note, SNO 2023 gave attendees in-depth opportunities to hear from world-renowned experts about the clinical impact and best practices for diagnosis and management of neurofibromatosis type 1 (NF1), a single-gene tumour predisposition syndrome with a diverse clinical presentation. This report will focus on the insights and data presented at SNO 2023 into the current state of the art in understanding and managing two key types of NF1-associated tumours: plexiform neurofibromas and cutaneous neurofibromas.
Chief Medical Editor: Dr. Léna Coïc, Montréal, Quebec
“Neurofibromatosis type 1 is, although a rare disease, the most common cancer predisposition syndrome worldwide. It affects about one in every 2,500 to 3,000 individuals, so that’s about 120 new diagnoses of patients in the US each day,“ explained Dr Laura Klesse, UT Southwestern Medical Center, Dallas, at the Education Day of SNO 2023. “This is a clear tumour predisposition syndrome – the cumulative risk of malignancy by age 50 is about 20 to 39%, which is a two- to ten-fold increase compared to the general population.” She explained that NF1 is associated with pathogenic variants in the NF1 gene, which are inherited in an autosomal dominant manner with complete penetrance but significant variability in the clinical presentation. Dr Klesse reviewed the characteristic clinical findings of NF1, which include:
- Skin pigmentation changes: café-au-lait macules (up to 90% of individuals with NF1), often at birth or early in childhood; skinfold freckling is also common (~75%)
- Ocular manifestations including Lisch nodules and choroidal abnormalities, both of which are generally asymptomatic
- Neurocognitive and/or social dysfunction (30-65%)
- Cutaneous and subcutaneous neurofibromas, present in the majority of adults with NF1
- Gliomas, typically low-grade optic pathway tumours (20-30%)
- Plexiform neurofibromas (30-50%); considered to be benign but with significant risks to the patient, including pain, disfigurement, loss of organ or limb function, and transformation into malignant peripheral nerve sheath tumours (MPNST)1
Dr Miriam Bornhorst, of the Gilbert Neurofibromatosis Clinic and George Washington University, Washington, DC, gave an overview of the pathophysiology of NF1 and potential targets for drug treatment. “NF1 is a tumour suppressor gene that keeps cells from growing when they’re not supposed to. So when you lose that function, you’re going to form a tumour, and depending on where you lose that function, you can have different types of tumours,” she said. She explained that when the NF1 gene is disrupted, there is impaired production of the tumour suppressor protein neurofibromin, which normally inhibits cell proliferation driven by the RAS-MAPK pathway and related cross-talk signalling pathways. Medications that target elements of RAS-MAPK and related pathways are therefore rational options to evaluate in NF1.2
Current state of the art for management of NF1-PN
“Although plexiform neurofibromas are histologically benign, they can cause a lot of problems and significant morbidity,” said Dr Andrea Gross, from the National Cancer Institute in Bethesda, Maryland. She explained that although surgical resection is possible in some cases, for many patients the location and/or architecture of their PNs makes surgery impossible, and even for patients who undergo resection, it is rarely complete and tumour regrowth is common.3
One option for treatment of inoperable PNs is selumetinib, an orally administered inhibitor of the MEK component of the RAS-MAPK pathway that has now been approved in Canada and many countries worldwide for management of inoperable NF1-associated PN in patients aged 2 and older. Participants at SNO 2023 had the opportunity to hear the history of selumetinib’s development from Dr Gross, one of the key investigators in the trial program. She described how the phase 1 trial, which was intended primarily to investigate the maximum tolerated dose of selumetinib in pediatric patients with inoperable PN, showed a surprising degree of tumour shrinkage in many patients. “We saw an exciting anecdotal benefit, but we didn’t have prospective functional measures included as part of the phase 1 trial,” Dr Gross said. “So that’s what led us to the development of the phase 2 registration trial for selumetinib, also known as SPRINT, where the primary objective was the overall response rate but we also had key secondary objectives of functional and patient-reported measures.”3
In SPRINT, 68% of patients had a confirmed partial response based on tumour volumetric analysis, and 72% of the pediatric patients and 86% of their parents reported subjective improvements in overall symptom burden. All patients experienced at least one adverse event (AE) but the large majority (97%) were mild (grade 1 or 2); the most common AEs were gastrointestinal events (diarrhea, nausea, vomiting) and skin and nail disorders (rash, paronychia).3,4
To further evaluate the safety and efficacy profile of selumetinib over an extended treatment period, Dr Gross and colleagues conducted a pooled analysis of long-term data from the phase 1 and 2 trials, which was published shortly before SNO 2023.5 ”We were able to look at an additional 45 cycles, so almost three-and-a-half to four years,” she explained. “We did not see any new safety signals but a lot of these patients are still on drug so we’ll continue to monitor them. The other thing that we found most reassuring, was that the improvements we originally saw in functional and patient-reported outcome measures – primarily in pain, for the patient-reported side – were maintained for patients still on treatment.”3 For patients on selumetinib, the median progression-free survival across the two trials was roughly 6.7 years, compared with 1.3 years for a similar cohort of untreated patients in a natural history study. (Figure 1)3,5
Figure 1. Over long-term follow-up, mean PFS with selumetinib treatment in NF1-PN was over 6 years
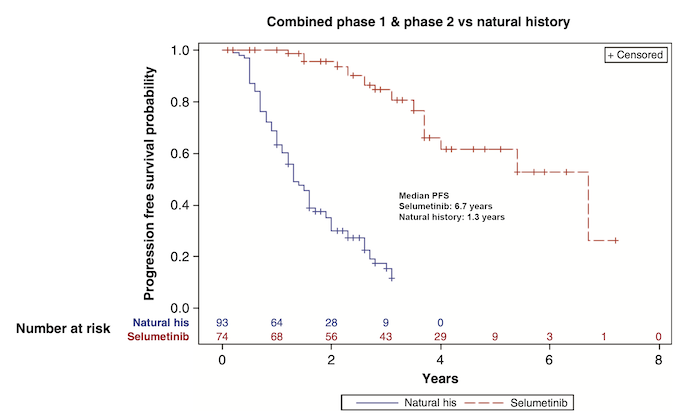
Adapted from: Gross AM, et al., Neuro Oncol 2023; 25(10):1883–1894.5
Advances in management of cutaneous neurofibromas
Cutaneous neurofibromas (CN) are a common skin manifestation of NF1. Although they are classified as benign tumours without any risk of malignant transformation, they can cause significant pain and itching, as well as cosmetic and quality-of-life challenges for patients. Until recently, treatment of CN has relied on surgery or other methods of physical tumour removal or reduction (e.g., CO2 laser, photocoagulation, radiofrequency ablation).6 Now, topical treatments are showing promise in relieving the symptoms of CN, as shown in two studies discussed at SNO 2023; neither of these investigational therapies is yet approved in Canada.
In a poster presentation, Dina Poplausky and colleagues from the Icahn School of Medicine at Mount Sinai, New York, described the results of their phase 1 study of topical diphencyprone in adults with CN. Diphencyprone is an immunotherapy agent that can alter the existing population of tumour-infiltrating immune cells; it has been used successfully in management of alopecia areata and cutaneous melanoma metastases. In this study, a 0.04% diphencyprone ointment was applied weekly for 10 weeks, followed by a recovery period, in 16 adults with NF-1 CN. Overall, patients noticed a subjective decrease in the size of small CN lesions, although no quantitative difference was detected on histological examination. The majority of adverse events were not attributable to treatment. The authors concluded that “Topical diphencyprone ointment is safe and tolerable in adults with NF1,” and said that ongoing and future studies would evaluate the changes in the tumour microenvironment with treatment and the depth of penetration of the drug into tumours.7
Several presenters additionally mentioned a press release that appeared several days before the conference, which described results from a randomized, vehicle-controlled phase 2b study of the topical MEK inhibitor NFX-179 in 199 patients with NF1-related CN. Compared with the vehicle alone, 44.2% of patients using a 1.5% gel formulation of NFX-179 once daily for six months achieved a statistically significant reduction in tumour size, defined as at least 50% reduction in CN volume above the surrounding non-tumour skin for five or more of the ten treated tumours.8 “These results are very exciting for the [cutaneous] neurofibromas,” said Dr Bornhorst, who was a lead investigator on the trial.6
Conclusions
Now that targeted therapies are becoming available that could change the disease course in some aspects of NF1, the key to optimal management of NF1 remains prompt and effective patient identification, as highlighted by Dr Klesse in her Education Day talk. “Identifying NF1 really matters, particularly for us as oncologists,” she said. “There are clear screening guidelines for NF1, and therapy decisions should be impacted by the presence of NF1. And then understanding the biological pathways is what’s driven the [development of] new therapies.”1
“I think we're in this really unique time and space – particularly in pediatric tumours – where not only are we understanding the pathogenesis and the drivers of these tumours, we actually have a bunch of drugs that target these pathways, which is a unique combination to have at one time,” said Dr Jason Fangusaro, Emory School of Medicine, Atlanta, in his summary of the session that discussed new medications for NF1-associated neurofibromas. “I think we're actually starting to come to the point where we're beginning to challenge some of the standard-of-care paradigms that we've used historically,” he concluded.9
References
1. Klesse L. Neurofibromatosis 1 Diagnosis and Clinical Features. Oral presentation during the Education Day of SNO 2023, November 15–19, 2023.
2. Bornhorst M. Targeted Therapies in Neurofibromatosis 1. Oral presentation during the Education Day of SNO 2023, November 15–19, 2023.
3. Gross AM. New Therapies in NF1-Associated Neurofibroma. Oral presentation at SNO 2023, November 15–19, 2023.
4. Gross AM, et al. N Engl J Med 2020; 382:1430–1442.
5. Gross AM, et al., Neuro Oncol 2023; 25(10):1883–1894.
6. Fisher M, et al. Tumors Associated With Neurofibromatosis Type 1: Precision Medicine Approaches to Improve Patient Outcomes Across All Age Groups. Symposium at SNO 2023, November 15–19, 2023.
7. Poplausky D, et al. Poster CTIM-04 at SNO 2023, November 15–19, 2023.
8. NFLection Therapeutics press release: NFlection Therapeutics Announces Positive Results from Phase 2b Study of NFX-179 Topical Gel in the Treatment of Cutaneous Neurofibromas in Neurofibromatosis Type 1. November 13, 2023. Available at: https://www.prnewswire.com/news-releases/nflection-therapeutics-announces-positive-results-from-phase-2b-study-of-nfx179-topical-gel-in-the-treatment-of-cutaneous-neurofibromas-in-neurofibromatosis-type-1-301986305.html
9. Fangusaro J. Introduction: New Drugs on the Market for Pediatric LGG and NF-Associated Neurofibroma. Oral presentation at SNO 2023, November 15–19, 2023.