Reports
“PNH is getting very popular!”: New clinical trial data and management insights from ASH 2023
von Riedemann, S., 2024. “PNH is getting very popular!”: New clinical trial data and management insights from ASH 2023. Mednet, https://doi.org/10.70270/na4pvys
This report is based on medical evidence presented at sanctioned medical congress, from peer reviewed literature or opinion provided by a qualified healthcare practitioner. The consumption of the information contained within this report is intended for qualified Canadian healthcare practitioners only.
PRIORITY PRESS - ASH 2023: Annual Meeting of the American Society of Hematology
San Diego, California / December 9–12, 2023
San Diego – Paroxysmal nocturnal hemoglobinuria (PNH) is a rare acquired disorder of hematopoietic stem cells that leaves red blood cells (RBCs) susceptible to complement-mediated lysis, with clinical manifestations that can include anemia, hemoglobinuria, and thrombosis. Inhibition of the terminal complement component C5 with eculizumab or ravulizumab can control intravascular hemolysis (IVH) and improve clinical parameters, patient survival, and quality of life (QoL). However, terminal complement inhibition alone may leave RBCs vulnerable to extravascular hemolysis (EVH) and persistent anemia in a minority of patients. This report focuses on clinical trial and real-world data presented at the recent annual meeting of the American Society of Hematology (ASH) for currently available and novel complement inhibitors in PNH.
Chief Medical Editor: Dr. Léna Coïc, Montréal, Quebec
“It’s nice to see a packed room – PNH is getting very popular!” remarked Dr Peter Hillmen of Apellis Pharmaceuticals to a standing-room-only audience at ASH 2023.1 In spite of its clinical rarity, PNH was a hot topic at the recent 65th ASH Annual Meeting, with more than 40 presentations including more than two dozen posters, four sponsored symposia, a Presidential Symposium on the complement system, and, for the first time ever, an entire oral session dedicated to clinical trials in PNH.
Several presenters remarked on how the introduction of the monoclonal antibody C5 inhibitor eculizumab 20 years ago and the subsequent development of ravulizumab, a next-generation product based on eculizumab, have revolutionized the management of PNH, providing many patients with a longer life expectancy, freedom from blood transfusions, and improved QoL. However, in spite of these advances, some patients still experience persistent hemolysis and anemia because C5 blockade can lead to deposition of C3 on RBCs, which triggers EVH. Several new strategies are therefore being developed for managing EVH and improving outcomes in patients with PNH for whom existing options are not sufficient.1,2
When asked about his overall impressions of the PNH content at ASH, Dr Christopher Patriquin, University Health Network, Toronto, and Chair of the Canadian PNH Network, said: “It’s just been a really exciting meeting, and really special to see so much PNH activity, and the diversity is incredible.”
New data for existing treatment options
The C5 inhibitor eculizumab, administered intravenously every 2 weeks, is the first-line standard of care in many parts of the world. Ravulizumab, designed to have an 8-week dosing interval, is now approved in Canada and several other countries, allowing for long-term analyses of safety and efficacy from clinical trials and registry data.
Dr Austin Kulasekararaj, King’s College Hospital, London UK, and colleagues, explored long-term outcomes from Study 301, the pivotal phase 3 trial that established the non-inferiority of ravulizumab versus eculizumab in previously untreated patients with PNH. They found that over more than 925 patient-years of follow-up, ravulizumab provided effective control of IVH and no new safety signals. In comparison to untreated patients in the International PNH Registry, patients on ravulizumab had longer overall survival. “The longest period of follow-up of C5 inhibitor-naïve patients treated with ravulizumab has been reported and compared with the Registry patients, supporting the use of ravulizumab as a first-line treatment of choice in patients with PNH,” concluded Dr Kulasekararaj.3
Dr Alexander Röth, University of Essen, Germany, and colleagues investigated real-world outcomes with ravulizumab among Registry patients switching from prior eculizumab. After switching, patients maintained control of IVH as measured by reductions in the key hemolysis marker lactate dehydrogenase (LDH, data not shown), and maintained their improvements in hemoglobin (Figure 1). Patients switched to ravulizumab did not experience any major adverse vascular events (MAVEs) or thromboembolic events (TEs), and required fewer transfusions (Figure 1).4
Figure 1. Long-term treatment with ravulizumab resulted in maintenance of hemoglobin levels and reduced rates of MAVEs, TEs, and RBC transfusion
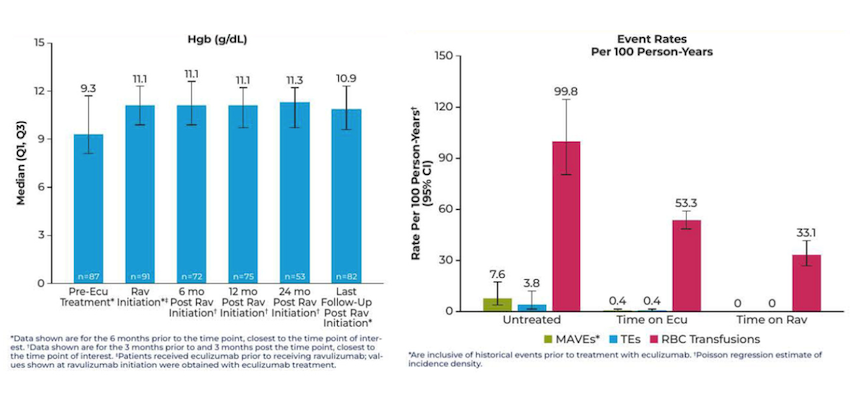
ecu, eculizumab; CI, confidence interval; MAVE, major adverse vascular event; rav, ravulizumab; RBC, red blood cell; TE, thromboembolic event.
Adapted from: Röth A, et al. Poster 2722 at ASH 2023, December 9–12, 2023.4
“I think it's really nice to see the longevity of the data with ravulizumab,” said Dr Patriquin, who was a co-author on the treatment switch poster. “We previously had non-inferiority studies [against eculizumab] but we're starting to be able to say, at least from observational data, that there seems to be further improvement – a reduction in patients who would qualify for high disease activity, transfusion avoidance is better, deeper LDH control, and theoretically protecting against the potentially devastating consequences of terminal complement not being fully suppressed.”
Long-term data were also presented for pegcetacoplan, a subcutaneously administered C3 inhibitor that was approved last year in Canada for second-line use in adults with PNH. Dr Carlos de Castro of Duke University in Durham, North Carolina, described how the improvements in hemoglobin and LDH seen in the initial stages of the PRINCE study (in C5-inhibitor-naïve patients) and the PEGASUS study (in C5-inhibitor-experienced patients) were maintained out to 2.5 and 3 years, respectively. Notably, transfusion burden was reduced throughout the long-term follow-up, with 67% of C5-naive patients and 52% of C5-experienced patients remaining transfusion-free throughout follow-up. “This integrated analysis shows that the efficacy of pegcetacoplan is robust and sustained in patients with PNH,” concluded Dr de Castro.5
Targeting unmet needs with new therapeutic approaches
Several presentations discussed new data for novel complement inhibitors; none of these agents are yet approved in Canada.
Danicopan
Dr Kulsasekararaj presented long-term data from the ALPHA phase 3 trial of danicopan, an orally administered inhibitor of the proximal complement component factor D, versus placebo as an add-on to eculizumab or ravulizumab in patients with PNH and clinically significant EVH. The main trial analysis, published recently in Lancet Neurology, showed that danicopan was superior to placebo for increasing mean hemoglobin levels from baseline, after 12 weeks of add-on treatment, with no new safety concerns.6 Following the initial randomized period, patients on placebo were switched to danicopan (plus background eculizumab or ravulizumab) and observed for an additional 12-week open-label phase plus an ongoing long-term extension. The hemoglobin improvement with danicopan was maintained out through 48 weeks of treatment; the proportion of patients who remained transfusion-free was maintained in patients who were on danicopan throughout all trial phases, and improved for patients previously on placebo who switched to danicopan. Adverse events were consistent with the safety profile in the 12-week analysis.7 In an additional analysis of patient-reported outcomes, danicopan add-on therapy was associated with improvements in fatigue and physical functioning, bringing these scores into the normal range of the general population.8
Iptacopan
Several presenters at ASH 2023 noted the recent approval by the US FDA of iptacopan, an oral inhibitor of factor B, in adults with PNH. Dr Antonia Risitano, University of Naples, Italy, gave two oral presentations on additional analyses of the pivotal phase 3 trials in treatment-naïve and treatment-experienced patients. In the final 48-week results from the APPLY-PNH phase 3 trial in previously treated patients with persistent anemia, iptacopan was able to increase hemoglobin to near-normal levels, with final values of 12.2 g/dL in patients who received iptacopan throughout the 48-week observation period and 12.1 g/dL for patients who switched from a C5 inhibitor to iptacopan after week 24. At week 48, over 90% of patients were transfusion-independent.9 Patient-reported outcomes across APPLY-PNH and the APPOINT-PNH trial in treatment-naïve patients showed clinically meaningful improvements in fatigue, health-related QoL, and disease symptoms.10
Expert perspective and key takeaways for Canadian clinicians
Asked about his key take-aways and advice for Canadian clinicians, Dr Patriquin said, “There is a lot more we still need to learn, but we know there are patients who aren't very well controlled on some drugs and who need something else. And having multiple options in the future is going to be great, but also challenging because we have to weigh all the pros and cons for each. Counseling [our patients] will be really important, and having open communication will be crucial, in terms of differential risks and side effect profiles and other clinical considerations that we've not really had to worry about with monoclonal antibodies alone.”
“As we get more novel therapies, there’s more and more interest in PNH,” said Dr Patriquin. “In the early days of eculizumab availability in Canada, once people heard about a drug, then they knew that they could look into testing [for PNH] more, because there was something that we can do about it. And I think that's going to be propelled even further with all of these different therapies. And identifying patients is still the key challenge – first we have to find them, and then we have to decide what to do, and that’s a discussion between patient and physician.”
References
1. Hillmen P. Apellis Product Theatre at ASH 2023, December 9–12, 2023.
2. Weitz, IC. Complement Wheel: Optimizing Care for Patients With aHUS, HSCT-TMA, and PNH. Sponsored symposium at ASH 2023, December 9–12, 2023.
3. Kulasekararaj A, et al. Poster 2714 at ASH 2023, December 9–12, 2023.
4. Röth A, et al. Poster 2722 at ASH 2023, December 9–12, 2023.
5. De Castro C, et al. Oral presentation 574 at ASH 2023, December 9–12, 2023.
6. Lee JW, et al. Lancet Haematol 2023 Dec;10(12):e955-e965.
7. Kulasekararaj A, et al. Oral presentation 576 at ASH 2023, December 9–12, 2023.
8. Piatek C, et al. Poster 1346 at ASH 2023, December 9–12, 2023.
9. Risitano A, et al. Oral presentation 571 at ASH 2023, December 9–12, 2023.
10. Risitano A, et al. Oral presentation 487 at ASH 2023, December 9–12, 2023.