Reports
A Long-Term Update with IL-17A Inhibition
This report is based on medical evidence presented at sanctioned medical congress, from peer reviewed literature or opinion provided by a qualified healthcare practitioner. The consumption of the information contained within this report is intended for qualified Canadian healthcare practitioners only.
MEDICAL FRONTIERS - 26th European Academy of Dermatology and Venereology Congress
Geneva, Switzerland / September 13-17, 2017
Geneva - Long-term data are emerging with the interleukin (IL)-17A inhibitors in the treatment of patients with moderate to severe plaque psoriasis and psoriatic arthritis. These findings, from open-label extensions of phase 2 and phase 3 studies, are proving strong evidence that the high Psoriasis Area and Severity Index 75, 90 and 100 responses observed early in the course of treatment are sustained out to as long as 5 years, with no new safety signals. Data from registries will help to clarify the impact of IL-17A inhibitors in the real world.
Chief Medical Editor: Dr. Léna Coïc, Montréal, Quebec
Interleukin (IL)-17A inhibitors are well established for the treatment of psoriasis as IL-17A is a key cytokine involved in the pathogenesis of psoriasis and psoriatic arthritis (PsA).
New data are emerging to demonstrate long-term maintenance of improvement in skin clearance with the IL-17A inhibitors. The sustainability of effect is evident with 5-year extension of phase 3 data now available with the fully human anti-IL-17A monoclonal antibody secukinumab in patients with moderate to severe psoriasis. Five years of efficacy and safety data from phase 2 clinical trials with ixekizumab, a humanized IgG4 monoclonal antibody that neutralizes IL-17, and brodalumab, a human, anti-IL17RA monoclonal antibody have also been generated.
The Rationale for Targeting IL-17
IL-17A is a proinflammatory cytokine that plays a role in host defense and immuno-inflammatory pathology. As such, IL-17A is an important mediator in plaque psoriasis as well as PsA. IL-17A therefore represents a rational target in the treatment of psoriasis and PsA because it is present in the epidermis and joints. “By targeting it, the benefit is rapid and robust,” said Dr. Richard Langley, Director of Research, Division of Dermatology at Dalhousie University, Halifax, Nova Scotia, Canada.
The level of clearance achieved with the IL-17A inhibitors in patients with psoriasis is unprecedented, with rapid onset of action: Psoriasis Area and Severity Index (PASI) 90 responses of 65% to 80% are achieved by week 16 of treatment. Higher PASI responses lead to greater improvements in quality of life, with the probability of achieving Dermatology Life Quality Index (DLQI) response increasing with a greater percentage change from baseline in PASI.
This rapid improvement in response is sustained. “We’re losing very few patients as we follow them out over time,” he said. “While we can look forward to making our patients happy in the short term [with the use of IL-17A antagonists], the long-term durable remission story is increasingly coming out. We’re seeing 5-year data coming out at this Congress and it’s reassuring and helpful when you’re counseling your patients.”
Open-label Extension of Phase 2 Studies
Ixekizumab. Efficacy, health-related outcomes, and safety of patients with chronic plaque psoriasis treated with ixekizumab for 5 years in an open-label extension of a phase 2 trial were reported by Dr. Andrew Blauvelt, Oregon Medical Research Center, Portland, USA. In the study, no safety/efficacy data exist with use of 80 mg of ixekizumab exclusively because patients were switched to this dose during the study once a decision was made to go forward with 80 mg in phase 3 trials.
After completing randomized treatment and washing out for 12 weeks, 120 of 192 patients entered the open-label long-term extension, and 67 (55.8%) completed at least 5 years of treatment. “Clinical response rates were stable throughout the extension,” said Dr. Blauvelt. The PASI 75 response rate was 86% at 52 weeks and remained at 83% among those completing 240 weeks of open-label treatment. Similar trends were observed for PASI 90 (74% at 52 weeks and 88% at 240 weeks) and PASI 100 (53% and 45%, respectively) responses.
Static Physician Global Assessment (sPGA) response rates with ixekizumab were maintained over 4.5 years of open-label treatment, by both the last observation carried forward method (imputed) and the observed data. An sPGA of 0-1 on the 5-point scale was achieved by 72% of patients at 52 weeks and by 64% at 240 weeks, and sPGA 0 was achieved by 54% and 48% at week 52 and week 240, respectively.
“Throughout the open-label extension, most treatment-emergent adverse events were considered mild or moderate overall,” said Dr. Blauvelt. The rate of treatment-emergent adverse events was 60.0% from week 1 to week 52 and 50.0% from week 208 to week 260.
Ixekizumab also provided sustained improvements in DLQI and itch reduction through 4.5 years of treatment. The mean change in DLQI from baseline to week 240 was -8.3 and the mean change in the itch visual analog scale from baseline to week 240 was -43.6.
Brodalumab. Rates of complete clearance were sustained out to about 5 years in an open-label extension of a 12-week dose-finding phase 2 study of brodalumab in patients with moderate to severe psoriasis. Patients enrolled in the 12-week study received either brodalumab (70/140/210 mg every 2 weeks with a loading dose at week 1, or 280 mg every 4 weeks) or placebo.
Some 181 patients entered an open-label extension in which all patients initially received brodalumab 210 mg every 2 weeks; a protocol amendment after about 1 year allowed patients weighing ≤100 kg to switch to 140 mg every 2 weeks (n=118), and a second amendment allowed patients with inadequate response to 140 mg to switch back to 210 mg every 2 weeks (n=30). Of the 181, 107 (59.1%) remained on treatment at week 264, with a median exposure of 264 weeks.
“The patients who had good control of their disease early in the course of treatment, at 12 weeks, they maintained their response at very high levels,” said Dr. Kim Papp, Probity Medical Research, Waterloo, Ontario, Canada.” Those who were at PASI 100 maintained their responses best over 5 years.”
At week 12, 62.9% of patients achieved a PASI 100, which persisted (59.4%) to week 240. The mean improvement in PASI was 95.4% at week 12 and 92.1% at week 240. At the final study visit (week 264), patients had been off treatment for at least 6 weeks, with a resultant decrease in response across all treatment groups.
Open-label Extension of Phase 3 Study
Secukinumab. The only phase 3 study for which 5-year extension data are available with an IL-17A antagonist is the multicentre SCULPTURE study that assessed secukinumab. Patients with moderate to severe plaque psoriasis who completed 52 weeks of double-blind treatment in SCULPTURE entered into a blinded extension period until week 156, followed by an open label extension phase until week 260. The primary objective of the extension study was to assess the long-term safety and tolerability of secukinumab. Efficacy measures included the proportion of patients who achieved PASI 75, PASI 90, and PASI 100 responses.
In SCULPTURE, patients who achieved a PASI 75 response at week 12 were randomized to double-blind maintenance treatment of secukinumab, 300 mg or 150 mg, given either at 4-week fixed intervals or retreatment as needed. The 642 patients who completed 52 weeks were eligible to continue with the same dose and regimen in the extension study.
Sustained efficacy was demonstrated in the 5-year extension (Figure 1). In the core study of 162 patients, PASI 75 and PASI 90 responses were achieved by 89% and 69% of patients, respectively, at 52 weeks. With 122 patients observed at 5 years, PASI 75 and PASI 90 responses were documented in 89% and 66%, respectively. Some 44% of patients achieved completely clear skin (PASI 100) at 1 year, a rate that was maintained to year 5 (41%).
Figure 1.
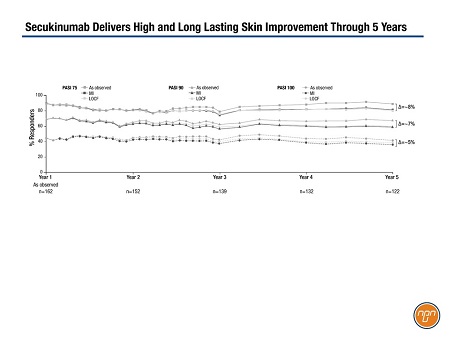
In addition to observed data, the analysis included multiple imputation. “Regardless of how you’re analyzing it, you’re seeing a long-term very sustained efficacy with this molecule,” said Dr. Langley. “Of course, not everybody continues to respond with any drug. We’re always going to have some drop-off but I think it’s reassuring when we’re seeing these kind of data.”
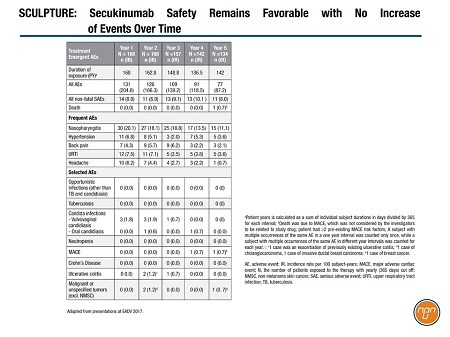
Secukinumab continued to have a favorable and consistent safety profile (Table 1), and low immunogenicity. Only 10 patients (6.6%) discontinued the extension because of adverse events. The most common adverse events continued to be nasopharyngitis, upper respiratory infection, and headache.
Table 1.
Registry Looks to Compare Long-term Outcomes in Real World
“Some of the best data we get on long-term safety are from registries but you also get very good information on durability of response,” said Dr. Langley.
Comparing long-term effectiveness between agents is problematic, however, because therapy is often optimized with dose and interval adjustments. Often, therapy has been adjusted after achievement of PASI 75 because patients desire an even better outcome, he said.
The prospective PURE registry of patients with moderate to severe chronic plaque psoriasis who were treated with secukinumab or other approved therapies in Latin America and Canada is targeted to enroll 2,500 patients from 90 community and hospital dermatology sites. The study includes a 5-year follow-up.
An interim analysis of 497 patients (186 on secukinumab and 311 on other treatment) shows that most patients had received other psoriasis treatment before their current treatment (91.9% secukinumab and 86.5% on other treatment; P=0.001). More patients being treated with secukinumab had received prior biologic treatment than patients on other treatment (60.8% vs. 20.3%; P=0.001).
Patients on secukinumab had worse historical PASI scores than those on other treatment (17.6 vs. 14.0; P=0.002). More than 80% of patients overall had a past or an ongoing comorbidity. A higher proportion of secukinumab-treated patients also had PsA than those on other treatment (18.8% vs. 10.9%; P=0.014).
Future analyses will focus on real-word safety, effectiveness, and health care utilization between secukinumab and other approved treatments but because of the significant differences in the characteristics of patients selected for treatment with each, "future analyses must therefore be interpreted with caution," concluded the registry's authors.
Questions and Answers
Questions and answers with Drs. Kim Papp, Probity Medical Research, Waterloo, Ontario, Canada and Richard Langley, Director of Research, Division of Dermatology at Dalhousie University, Halifax, Nova Scotia, Canada.
Q: The IL-17A inhibitors are now well established. How has their introduction changed the standard of care for patients with psoriasis?
Dr. Papp: There has been a quantum jump in our expectations to achieve higher levels of clearance. At the time the PASI 75 target was established, which was almost 2 decades ago, it was a reasonable threshold. It was the gauge by which we were measuring response because most of the drugs that we were using, such as the tumor necrosis factor (TNF) inhibitors couldn’t achieve much better than PASI 75 responses. The prime directive when the IL-17As were developed was finding an optimal dose to treat psoriasis. They could push for higher exposures, which led to much higher efficacy compared to the TNF antagonists.
Dr. Langley: We recognize that the IL-23/Th17/IL-17A pathway is the critical pathway in regulating psoriasis. The rapid, robust, and sustained improvement in psoriasis with the IL-17A inhibitors observed in clinical trials has helped confirm that understanding.
Also, recent data from ixekizumab and in the FUTURE studies with secukinumab have shown efficacy in psoriatic arthritis (PsA) that has rivaled that of the TNF antagonists, which to this point have been the gold standard for treatment of PsA.
Q: What is the biggest impact on standard of care for these patients in the real world?
Dr. Langley: Achievement of PASI 90 and PASI 100 is significant with IL-17 inhibitors. Being essentially clear or almost clear is meaningful to patients. It has been shown that it correlates highly with the Dermatologic Quality of Life Index. Patients are essentially telling us that they have a normal quality of life, and some can now wear shorts or clothes that reveal their arms.
Q: Why are 5-year data important for physicians?
Dr. Papp: Five-year data are important to physicians treating psoriasis with any of the biologics because the 5-year data set the tone for the durability of response and the ability of a particular agent to not only maintain response but to maintain a high level of response. At 5 years, you also have a robust test of safety.
Please comment on the importance of registry data, in particular, the value of the data found in PURE.
Dr. Papp: Even though inclusion criteria for clinical trials are more liberal now than they were 5 or 6 years ago, they are still highly regulated. The registry data help on two fronts.
They consolidate or complement the results from the clinical trials. It gives us a little more insight into the real-world safety signals, when you’re throwing in a mix of patients who have pre-existing diseases and comorbidities. The registry also provides a real-world view of durability.