Reports
Update on the Impact of IL-17A Inhibition on Disease Severity and Structural Changes in Axial Spondyloarthritis
This report is based on medical evidence presented at sanctioned medical congress, from peer reviewed literature or opinion provided by a qualified healthcare practitioner. The consumption of the information contained within this report is intended for qualified Canadian healthcare practitioners only.
MEDICAL FRONTIERS - Annual Meeting of the American College of Rheumatology
San Diego, California / November 4-7, 2017
San Diego - Joint symptoms and structural joint damage are two targets of the treatment of axial spondyloarthritis (axSpA), which encompasses psoriatic arthritis and ankylosing spondylitis. Inhibition of interleukin-17A, a key cytokine involved in the pathogenesis of axSpA, improves the symptoms of disease while slowing its radiographic progression. Recent findings support that the improvements are sustained with follow-up to 4 years.
In addition to the impact of disease activity on reduced function in psoriatic arthritis (PsA) and ankylosing spondylitis (AS), erosive structural damage also influences function and spinal mobility. Treatments that address the disease on a structural level by slowing the progression of joint damage may therefore offer benefits beyond reducing the severity of joint pain and stiffness. Data indicate that interleukin (IL)-17A inhibitors not only favorably affect the signs and symptoms of AS and PsA but inhibit the progression of joint structural damage.
Low Radiographic Progression in AS at 4 Years
Structural progression in AS is characterized by new bone formation, and can lead to ankylosis of the sacroiliac joints (SIJ) and the spine. Disease-modifying therapies that can reduce the rate of structural progression represent an unmet need, said Dr. Jürgen Braun, Medical Director, Rheumazentrum Ruhrgebiet, Herne, Germany.
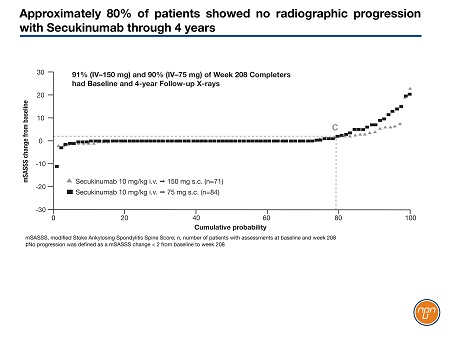
In the MEASURE 1 study, sustained improvements in the signs and symptoms of AS through 2 years were realized with treatment using the fully human anti-IL-17A monoclonal antibody secukinumab. In addition, about 80% of patients showed no radiographic structural disease progression.
“We have a clear-cut demonstration of efficacy of the 150-mg [approved] dose through 4 years in the MEASURE 1 study,” said Dr. Braun. “We had no progression in the majority of patients.”
Data from the MEASURE 1 extension trial support maintenance of the efficacy of secukinumab on symptoms and radiographic progression to 4 years (208 weeks). In MEASURE 1, 371 patients with active AS were randomized to intravenous secukinumab (10 mg/kg) or matched placebo at weeks 0, 2, and 4, followed by subcutaneous secukinumab (150 mg or 75 mg) or matched placebo every 4 weeks starting at week 8. Patients on placebo who met Assessment of Spondyloarthritis International Society (ASAS) 20 response criteria at week 16 switched to secukinumab at week 24. Nonresponders could receive escape treatment with either 75 or 150 mg of secukinumab at week 16, and then every 4 weeks.
Radiographic progression was measured by lateral radiographs of the cervical and lumbar spine performed at baseline and at weeks 104 and 208. Digitized images for each patient were scored by the same reader using the modified Stoke Ankylosing Spondylitis Spine Score (mSASSS).
At 3 years, as previously reported, 79.5% and 60.9% of patients treated with 150 mg of secukinumab achieved ASAS20 and ASAS40 response criteria, respectively. From baseline to 3 years, the mean change in the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) was -3.1, the mean change in the Bath Ankylosing Spondylitis Functional Index (BASFI) was -2.8, and the mean change in the Bath Ankylosing Spondylitis Metrology Index (BASMI) was -0.7, with all changes similar to those at 2 years.
At 4 years, 79.7% achieved an ASAS20 response and 60.8% an ASAS40 response (observed data) “showing a sustained clinical response to secukinumab,” said Dr. Braun. Through week 208, changes from baseline were -3.4 on the BASDAI, -2.9 on the BASFI, and -0.5 on the BASMI.
The mean mSASSS change from baseline to year 2 with any secukinumab dose, as previously reported, was 0.30. There was sustained low progression in mSASSS at year 4 with either the 75-mg or 150-mg secukinumab dose; the mean change in the mSASSS from baseline to year 4 was 1.2 with the 150-mg dose. Secukinumab, 150 mg, was associated with a numerically lower rate of progression versus 75 mg at 4 years. These are the first data from a controlled study of a biologic in AS to suggest a dose-response effect in inhibiting structural progression, Dr. Braun noted. Some 78.9% of patients had no radiographic progression (defined as a mSASSS change <2 from baseline) at year 4 with secukinumab (Figure 1).
Figure 1.
Over the 4 years, 32 (8.9%) of 360 patients discontinued secukinumab due to adverse events. Serious adverse events included 21 patients with uveitis, four of whom had a history of uveitis; seven with Crohn disease, two with a history of Crohn disease; and two with ulcerative colitis.
Effect on Structural Disease in PsA
Radiographic progression of structural joint damage was inhibited in patients with PsA who were treated with secukinumab, especially in those treated with a loading dose.
In the FUTURE 5 study, a significantly higher proportion of patients randomized to secukinumab showed no worsening of structural joint damage at 24 weeks compared with those randomized to placebo, according to Dr. Philip J. Mease, Director, Division of Rheumatology Clinical Research, University of Washington, Seattle, USA. The safety profile of secukinumab was consistent with that previously reported, with no new safety signals.
Secukinumab is the second agent in the IL-17A inhibitor class to demonstrate inhibition of radiographic progression of PsA. In the SPIRIT-P1 study, for which Dr. Mease was also the lead investigator, ixekizumab was superior to both placebo and adalimumab on measures of structural damage progression in patients with PsA who were not previously treated with biologic agents, whereas FUTURE 5 included both patients who were naïve to tumor necrosis factor (TNF) inhibitors and those with inadequate response to a TNF inhibitor.
With two drugs in the IL-17A inhibitor class showing a benefit on structural disease in PsA, “it’s confidence building,” said Dr. Mease. “Over the long haul, we can expect to have some inhibition of radiographic progression.”
In FUTURE 5, 996 adults with active PsA were randomized to one of four groups: secukinumab, 300 mg with a 300-mg loading dose; secukinumab, 150 mg with or without a 150-mg loading dose; or placebo. All groups received secukinumab or placebo at baseline and then at 1, 2, 3 and 4 weeks, and then every 4 weeks thereafter.
A ≥20% improvement from baseline in the ACR response criteria at week 16, the primary endpoint, was achieved by 62.6% of the group receiving 300 mg of secukinumab with a loading dose, compared with 27.4% of the placebo group (P<0.0001). Some 55.5% of patients receiving 150 mg of secukinumab with a loading dose and 59.5% of those receiving 150 mg of secukinumab without a loading dose achieved an ACR20 response (P<0.0001 for both secukinumab 150 mg groups).
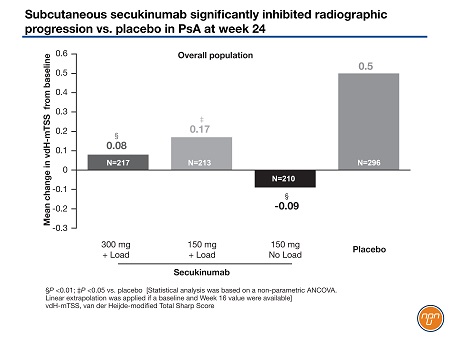
Radiographic structural progression was determined by the change from baseline in the mean van der Heijde-modified Total Sharp score (vdH-mTSS). At week 24, 88% of patients assigned to 300 mg of secukinumab had no radiographic progression compared with 80% of the patients assigned to 150 mg of secukinumab with a loading dose, 84% of those randomized to 150 mg of secukinumab without a loading dose, and 74% of patients randomized to placebo.
Mean changes in the vdH-mTSS at week 24 in the overall population (Figure 2) were 0.5 in the placebo group and 0.08 in those randomized to 300 mg of secukinumab (P<0.01), 0.17 in those randomized to 150 mg of secukinumab with loading dose (P<0.05 vs. placebo) and -0.09 in those randomized to 150 mg of secukinumab with no loading dose (P<0.01 vs. placebo). In the patients naïve to a TNF inhibitor, the changes in the vdH-mTSS were 0.48 in the placebo arm, 0.01 in the secukinumab 300 mg arm (P<0.001), 0.12 in the secukinumab 150 mg with a loading dose arm (P<0.05 vs. placebo) and -0.25 in the arm that received secukinumab 150 mg without a loading dose (P<0.05 vs. placebo).
Figure 2.
Some 61.4% of the secukinumab 300-mg group had resolution of enthesitis, which was significantly better than the 34.4% in the placebo group (P<0.0001). Secukinumab 150 mg with (P<0.05) and without (P<0.001) a loading dose were also significantly better than placebo on this endpoint. As well, complete resolution of dactylitis was significantly more common in all secukinumab dose groups compared with placebo (P<0.0001 for all secukinumab arms vs. placebo).
“The data tell me that you need to use ideally 300 mg and you need the load; five injections at the beginning followed by monthly dosing,” he said.
Data on radiographic progression of structural joint damage over 52 weeks in patients with active PsA who were treated with ixekizumab were presented by Dr. Désirée van der Heijde, Professor of Rheumatology, Leiden University Medical Center, The Netherlands. The 417 patients enrolled into the double-blind phase 3 study were randomized to ixekizumab, 80 mg every 2 or 4 weeks following a 160-mg loading dose, placebo, or adalimumab, 40 mg every 2 weeks for 24 weeks. In the extension phase of the study, 381 patients initially randomized to placebo or adalimumab were re-randomized to ixekizumab every 2 or 4 weeks. Patients were assessed for structural joint damage using the vdH-mTSS.
The mean changes in mTSS from baseline to week 52 were 0.54 and 0.09 for patients randomized to ixekizumab every 4 weeks and every 2 weeks, respectively, at baseline. Most patients exhibited no structural progression through 1 year of ixekizumab treatment.
Predictors of Structural Damage and Response to Treatment
Local inflammation in the SIJ and spine as detected by MRI is associated with the development of MRI structural damage over 5 years in early axial spondyloarthritis (axSpA), found investigators at Leiden University Medical Center, The Netherlands. In their study, 155 patients with axSpA of 3 years’ or less duration had MRI assessments at baseline and again at 5 years. The presence of bone marrow edema in the SIJ and spine at baseline were highly predictive of finding at least 3 fatty lesions 5 years later at both sites. Patients who were positive for bone marrow edema in the SIJ at baseline had a higher probability of fatty lesions (net structural progression) compared with patients without baseline bone marrow edema. The effect was independent of systemic inflammation, as determined by levels of high-sensitivity C-reactive protein.
In the phase 3 ASTREA study, a higher degree of baseline structural joint damage and higher swollen joint count predicted a greater response to abatacept therapy in patients with PsA, according to a team of researchers led by Dr. Georg Schett, Professor and Chair, Department of Medicine, Rheumatology and Immunology, University of Erlangen-Nuremberg, Germany. The total Sharp/van der Heijde scores were higher in patients who achieved an ACR50 response (84.0) and an ACR70 response (75.8) compared with those who achieved an ACR20 response (36.6). Those achieving ACR50 and ACR70 responses had a mean of 8.5 and 8.0 swollen joints, respectively, compared with a mean of 7.0 swollen joints in patients who achieved an ACR20 response. “These observations suggest that PsA patients with signs of synovitis-driven osteoclast formation and disease activity including swollen joints and structural damage may be good candidates for abatacept treatment,” the researchers concluded.
Achievement of Remission
Two-year results from the 5-year randomized, double-blind, multicenter, placebo-controlled phase 3 FUTURE 2 study showed that a higher proportion of patients with PsA assigned to secukinumab achieved a state of remission or low disease activity compared with placebo, consistent with the results obtained at week 16. At 2 years, 29% of patients treated with secukinumab, 300 mg, and 17% assigned to secukinumab, 150 mg, achieved Disease Activity Index for Psoriatic Arthritis (DAPSA)-remission, an improvement over 14% and 10%, respectively, at week 16, reported Dr. Laura Coates, clinical lecturer in Rheumatology, University of Oxford, Leeds, Great Britain. In the placebo group, only 5% reached DAPSA-remission at week 16 (placebo patients were re-randomized at week 16 or 24 to secukinumab).
A comparative effectiveness retrospective analysis of secukinumab, 150 mg, and golimumab, 50 mg, in biologic-naïve patients with AS showed evidence of better response with secukinumab in non-placebo-adjusted comparisons at 24 weeks, reported Dr. Walter Maksymowych, Professor of Medicine, University of Alberta, Edmonton, Canada.
Patients in the analysis were chosen from the MEASURE 1 and MEASURE 2 trials with secukinumab and the GO-RAISE study with golimumab. Individual patient data from the pooled secukinumab 150-mg arms of MEASURE 1 and MEASURE 2 (n=197) were weighted to match the baseline characteristics of the 138 patients in the golimumab arm of GO-RAISE. The placebo arms of MEASURE 1 and MEASURE 2 were also matched to the placebo arm of GO-RAISE.
In placebo-adjusted comparisons, there was no difference in ASAS20, ASAS40, and ASAS partial remission response rates between secukinumab and golimumab at weeks 12/14 and 14/16. In non-placebo-adjusted comparisons, at week 24, ASAS20 and ASAS40 response rates were numerically higher with secukinumab versus golimumab, but the differences were not significant (ASAS20 OR: 1.58; P=0.089 and ASAS40 OR: 1.58; P=0.084).
Questions and Answers
Questions and answers with Dr. Walter Maksymowych, Professor of Medicine, University of Alberta, Edmonton, Canada
Q: What are your thoughts on the MEASURE 1 4-year radiographic progression data presented at ACR?
A: This follow-up of the 4-year data from the MEASURE 1 trial has shown continuing low radiographic progression in patients with AS treated with secukinumab and a lower rate in those receiving the 150 mg dose monthly versus those receiving 75 mg monthly. This is an interesting new study design to assess radiographic progression in patients with this disease. It should be noted that the P value for this comparison was not provided so it is unclear if the difference between the two dosing arms was statistically significant. In addition, an analysis adjusted for potential differences in baseline characteristics that might impact radiographic progression (e.g., baseline mSASSS) was not presented. The results should be regarded as preliminary until these key data from the analysis are made available.
Q: Why is inhibition of radiographic progression of disease important to patients? Will it have an impact on disability? Can it be used to make an argument for earlier treatment?
A: Prevention of radiographic progression is important to patients because when they research the disease they find pictures on the internet of patients with severe kyphosis and so they want to avoid this outcome. Spinal ankylosis has a major impact on disability and we should continue to look for ways to prevent this with treatment. Treatment should still be based primarily on the severity of symptoms but increasingly, clinicians should be thinking of introducing more potent anti-inflammatory therapies when patients demonstrate evidence of prognostic factors associated with radiographic progression.
Q: In which PsA patients would you use ultrasound or MRI to measure structural disease and its progression?
A: We still do not yet have validated methods by which we can use ultrasound or MRI to measure structural progression in PsA.