Reports
Centre hospitalier universitaire de Sherbrooke
This report is based on medical evidence presented at sanctioned medical congress, from peer reviewed literature or opinion provided by a qualified healthcare practitioner. The consumption of the information contained within this report is intended for qualified Canadian healthcare practitioners only.
PHYSICIAN PERSPECTIVE - Get with the PROTOCOL - A Regional Perspective on the Evidence and Resulting Changes in ACS Protocol
April 2013
Reviewed and edited by:
Michel Nguyen, MD, FRCPC
Interventional Cardiology
Centre hospitalier universitaire de Sherbrooke
Professor of Medicine
Université de Sherbrooke
Sherbrooke, Quebec
Introduction
Antiplatelet therapy in the management of acute coronary syndromes (ACS) has recently been revised at the Centre hospitalier universitaire de Sherbrooke (CHUS). The altered algorithm is designed to provide significant reductions in risk of major cardiovascular (CV) events by employing the newer antiplatelet agents compared to the previous standard of clopidogrel and ASA. The new algorithm specifies where prasugrel and ticagrelor should be employed to provide additional protection against major CV events. Guided by the large clinical trials that underlie the revised algorithm, the specific recommendations allow for the clinical gains from greater antiplatelet effect, less recurrent ischemic events, including in some cases, a lower risk of death. They also balance benefit with an acceptably low increased risk of major or minor bleeding. Due to the fundamental importance of deactivating platelets to alter the natural history of evolving ACS events, optimal use of antiplatelet therapy should be considered an essential strategy for improving the prognosis of ACS. The new protocol is consistent with revisions in national guidelines.
Previous Standard: Need for Improvement
The dual antiplatelet strategy of clopidogrel and ASA in patients presenting with ACS has been a widely employed standard for more than 10 years. However, rates of CV events in ACS populations remain substantial. In the landmark CURE study, 10% of those receiving clopidogrel plus ASA went on to a recurrent myocardial infarction (MI), stroke or died of a CV cause despite the 20% reduction with the combination relative to ASA alone.1 This study was performed in patients with non-ST elevation MI (NSTEMI). In the CLARITY-TIMI 28 trial, conducted in patients with ST elevation MI (STEMI), the residual risk of death, stroke or MI was 9% in the group receiving clopidogrel plus ASA despite a 31% reduction in MI relative to ASA alone.2
Since those studies established this dual antiplatelet combination as a standard in ACS, two large ACS trials have proven that more effective antiplatelet therapy will further reduce CV risk. One trial tested ticagrelor in an all-comer population of ACS patients including those on clopidogrel before enrolment.3 The other study tested prasugrel in ACS patients, clopidogrel-naive, scheduled for a percutaneous coronary intervention (PCI).4 Data from these trials provide an opportunity to improve outcomes over the previous clopidogrel plus ASA standard.
In the TRITON TIMI-38 study, 13,608 ACS patients scheduled for PCI or eligible for ad hoc PCI were randomized. In the experimental arm, patients received a loading dose of prasugrel (60 mg) followed by maintenance prasugrel (10 mg daily). The comparator arm received a loading dose of clopidogrel (300 mg) followed by maintenance clopidogrel (75 mg daily). In both arms loading was performed mainly just before PCI. Both groups received ASA. Approximately 25% of the ACS events were STEMI and the remaining NSTEMI.
Relative to clopidogrel, prasugrel reduced the risk of the composite end point of death from CV cause, MI or stroke by 19% (HR 0.81; P<0.001), mainly driven by MI reduction. The risk of major bleeding on prasugrel was increased by 32% (HR 1.32; P=0.03) and life-threatening bleeding by 52% (HR 1.52; P=0.01) relative to clopidogrel. There was no difference in mortality. The authors concluded that the greater protection against recurrent ischemic events must be weighed against an increased risk of bleeding, but post-hoc analyses, with inherent limitations, provided guidance for candidate selection. In TRILOGY-ACS, prasugrel did not show benefit over clopidogrel in medically-treated subjects.5
In the PLATO trial, all individuals admitted to hospital with ACS were randomized regardless of planned procedure or pre-hospital antiplatelet treatment. Inclusion was early allowing upstream treatment, which was consistent with regional and national established protocols and guidelines in NSTEMI. The experimental arm received a loading dose of ticagrelor (180 mg) followed by maintenance ticagrelor (90 mg twice daily). The comparator arm received a loading dose of clopidogrel (300 or 600 mg) followed by maintenance clopidogrel (75 mg daily). Patients on clopidogrel before could be enrolled and switched or continued on according to the randomization with possible reloading concordant with arm assignment. Both groups received ASA. Approximately 37% of the 18,624 patients randomized had STEMI and the remaining had NSTEMI.
Relative to clopidogrel, ticagrelor reduced the risk of the composite end point of death from vascular causes, MI or stroke by 16% (HR 0.84; P<0.001). The difference in total major bleeding (11.6% vs. 11.2%; P=0.43) did not reach statistical significance but non-CABG major bleeding was significantly increased (4.5% vs. 3.8%; P=0.026). Unique to antiplatelet trials, ticagrelor was associated with a 22% reduction (HR 0.78; nominal P<0.001) in all-cause mortality (death from vascular causes 21%, HR 0.79; nominal P<0.001). A consistent advantage of ticagrelor was seen across nearly all subgroups including subjects with antecedents of transient ischemic attacks (TIA) or stroke. This advantage was independent of age, weight or management strategy (early invasive, selective invasive or conservative/medical).
New Data Translated into Clinical Practice
The revised treatment algorithms for antiplatelet therapy in ACS patients are designed to capture the opportunity to improve outcome based on the PLATO and TRITON-TIMI 38 trials. While all ACS patients should be initiated on ASA immediately, the second antiplatelet agent is defined by the diagnosis, the planned strategies for intervention and specific patient characteristics. Several large organizations have altered ACS antiplatelet guidelines on the basis of the PLATO and TRITON-TIMI 38 trials, but algorithms at the regional or hospital level are appropriate because of differences related to established protocols and network organization for ACS care.
At regional centres, including the CHUS, the data generated by the PLATO and TRITON-TIMI 38 trials can be applied directly. In NSTEMI patients, ticagrelor is now the dominant partner in a dual antiplatelet strategy with ASA with important exceptions. These exceptions include patients on an anticoagulant or who have had a prior intracranial hemorrhage (Figure 1). In those not candidates for ticagrelor, clopidogrel remains the preferred partner with ASA. In those initiated on clopidogrel who are candidates for ticagrelor according to the algorithm, the therapy should be switched.
Figure 1.
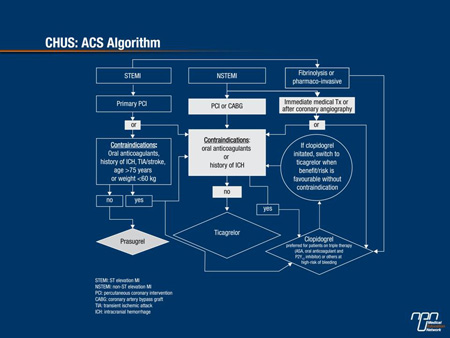
In STEMI patients scheduled for PCI, prasugrel has been found more effective than clopidogrel in a dual antiplatelet strategy with ASA, but the benefit-to-risk ratio varied across subpopulations in the main registration trial, producing some important exceptions and limitations. As a result, clopidogrel compared to prasugrel, not only remains the preferred partner with ASA in individuals who have received anticoagulants, with antecedent of intracranial hemorrhage or recent treatment with a fibrinolytic agent but also in those over the age of 75 years, those who weigh <60 kg and those with a prior TIA or stroke. In these individuals, the evidence suggests that the greater risk of major bleeding negates the clinical benefit provided by the relative reduction in CV events.
Ticagrelor on the other hand is an attractive alternative to clopidogrel in STEMI patients without oral anticoagulants or antecedents of intracranial hemorrhage scheduled for primary PCI.
Except for clopidogrel allergy, the therapeutic range of newer antiplatelets is limited to ACS up to 12-15 months after index event. There is no indication for the new agents long term in stable CAD or for elective placement of cardiac stent where clopidogrel is still recommended.
In all patients but especially when dual antiplatelet therapy with newer agents is prescribed, low dose ASA (80-160 mg) should be used to optimize the benefit-to-risk ratio.
Relevance of New Algorithm to Regional Centres
The objective evidence that newer antiplatelet therapies can improve the outcome in ACS patients informs but does not dictate adjustments in patient care. Due to substantial regional disparities in the care of ACS driven largely by variability in resources, such as the proximity of rapid response teams and differences in transfer intervals to catheterization laboratories, treatment guidelines must be adjusted for relevance to current practice. At regional centres, treatment algorithms must be adjusted for these variables. The same opportunities to improve outcome with more effective antiplatelet regimens should not be overlooked. Current practice at regional facilities is relevant to community hospitals whether or not the transfer of patients is common. Early upstream treatment gives opportunities to catch early benefits and maximizing advantages.
The new guidelines at CHUS include several assumptions that may or may not be relevant to nearby community centres. The protocol and algorithm applied is consistent with current national guidelines. However, the revised algorithm is evidence-based and does specifically outline areas in which clopidogrel plus ASA should no longer be considered the standard.
There are compelling data to conclude that implementation of more modern strategies for appropriate candidates will improve ACS outcomes including a reduction in mortality. The implementation and adherence to treatment guidelines in the management of ACS has been associated with statistically significant improvements in outcome. In an observational analysis that included 350 academic and non-academic centres, a stepwise 10% reduction in in-hospital mortality rates was associated with each 10% increase in adherence to evidence-based guidelines.6
Conclusion
The first-line antiplatelet strategies in ACS patients have been revised. The newer agents ticagrelor and prasugrel provide an important opportunity to improve outcome relative to clopidogrel when any of these agents is combined with ASA. In NSTEMI and STEMI patients, the advantage of ticagrelor over clopidogrel in appropriately selected patients includes a mortality reduction. The guidelines developed by CHUS were designed specifically to identify these opportunities in a readily applied algorithm.
Questions & Answers
Q: What is your perspective on the benefit-to-risk ratio that the newer antiplatelet agents offer within the revised algorithm for reducing the risk of thrombosis within an acceptable rate of bleeding?
A: The benefit-to-risk ratio favours ticagrelor more than prasugrel as excess bleeding risk is more modest with ticagrelor and there are fewer subgroups in which there is a risk that bleeding will blunt the benefit-to-risk.
Q: The studies that led to changes in the guidelines compared therapies in different populations. What insights can you offer on why it was important to prove superiority of prasugrel or ticagrelor over clopidogrel in different ACS groups (STEMI, NSTEMI, unstable angina, etc)?
A: Both prasugrel and ticagrelor have been studied in different ACS groups but the greatest supportive evidence for their use is derived from NSTEMI and STEMI and both settings have different physiopathology, management strategy and outcome. The ticagrelor advantage is present in nearly all subgroups including those with a history of cerebrovascular ischemic events, those who have been medically-treated, or those who have undergone an invasive strategy. There is no difference in benefit related to age or weight. In TRILOGY ACS, prasugrel offered no advantage in medically-treated patients compared to clopidogrel.5
Q: What is your point of view on the possible side effects associated with the newer agents vs. the opportunity to improve outcomes?
A: The main concern is risk of bleeding. However, the increased risk of non-CABG bleeding is only modest, particularly with ticagrelor, and judicious selection of patients helps to optimize benefit. The 2 other major potential adverse events with ticagrelor are dyspnea and bradyarrhythmia. The former is benign and easily distinguishable from heart failure or COPD decompensation, with the reassurance that medication can usually be continued. In PLATO, bradyarrythmia did not lead to a clinically significant event. However, the risk of these adverse events should be considered for patient selection and during follow-up.
Q: The algorithm introduces some decision points not required when all patients were treated with clopidogrel plus ASA. What action needs to be taken to improve outcomes?
A: Knowledge translation may facilitate implementation.
References
1. Yusuf et al. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med 2001;345(7):494-502.
2. Sabatine et al. Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST-segment
elevation. N Engl J Med 2005;352(12):1179-89.
3. Wallentin et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2009;361(11):1045-57.
4. Wiviott et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007;357(20):2001-15.
5. Roe et al. Prasugrel versus clopidogrel for acute coronary syndromes without revascularization. N Engl J Med 2012;367:1297-309.
6. Peterson et al. Association between hospital process performance and outcomes among patients with acute coronary syndromes. JAMA 2006;295(16):1912-20.