Reports
Centre hospitalier universitaire de Québec
This report is based on medical evidence presented at sanctioned medical congress, from peer reviewed literature or opinion provided by a qualified healthcare practitioner. The consumption of the information contained within this report is intended for qualified Canadian healthcare practitioners only.
PHYSICIAN PERSPECTIVE - Get with the PROTOCOL - A Regional Perspective on the Evidence and Resulting Changes in ACS Protocol
April 2013
Reviewed and edited by:
Patrick Béliveau, MD, CSPQ, FRCPC
Cardiologue
Hôtel Dieu de Québec-CHU de Québec
Québec, Québec
Introduction
The management of acute coronary syndromes (ACS) has recently been improved by arrival of the newer antiplatelet agents compared to the previous standard of clopidogrel and ASA. Where prasugrel and ticagrelor can be employed, we can provide additional protection against major CV events. Guided by the large clinical trials, the specific recommendations allow for the clinical gains from greater antiplatelet effect, including in some cases, a lower risk of death. They also balance benefit with an acceptably low risk of major or minor bleeding. Due to the fundamental importance of deactivating platelets to alter the natural history of evolving ACS events, optimal use of antiplatelet therapy should be considered an essential strategy for improving the prognosis of ACS.
Previous Standard: Need for Improvement
The dual antiplatelet strategy of clopidogrel + ASA in patients presenting with ACS has been a widely employed standard for more than 10 years. However, rates of CV events in ACS populations remain substantial. In the landmark CURE study, 9.3% of those receiving clopidogrel + ASA went on to a recurrent myocardial infarction (MI), a stroke or died of a CV cause despite the 20% reduction with the combination relative to ASA alone (Yusuf et al. N Engl J Med 2001;345(7):494-502). This study was performed in patients with unstable angina and non-ST elevation MI (NSTEMI). In the CLARITY-TIMI 28 trial, conducted in patients with ST elevation MI (STEMI), the residual risk was 9% in the group receiving clopidogrel + ASA despite a 31% reduction in risk of a major CV event relative to ASA alone (Sabatine et al. N Engl J Med 2005;352(12):1179-89).
Since those studies established this dual antiplatelet combination as a standard in ACS, 2 large ACS trials have proven that more effective antiplatelet therapy will further reduce CV risk. One trial tested ticagrelor in an all-comer population of ACS patients (Wallentin et al. N Engl J Med 2009;361(11):1045-57). The other study was designed to test prasugrel in ACS patients scheduled for a percutaneous coronary intervention (PCI) (Wiviott et al. N Engl J Med 2007;357(20):2001-15; Montalescot et al. Lancet 2009;373:723-31). The study drug was started before the placement of the first coronary guidewire in 25% of patients and during or within 1 hour of the PCI in almost all of the others. Data from these trials provide an opportunity to improve outcomes over the previous clopidogrel/ASA standard.
In the TRITON TIMI-38 study, 13,608 ACS patients scheduled for PCI were randomized to a 60 mg loading dose of prasugrel followed by a 10 mg daily maintenance dose or a 300 mg loading dose of clopidogrel followed by a 75 mg daily maintenance dose. Both groups received ASA. Approximately 25% of the ACS events were STEMI and the remaining NSTEMI. Relative to clopidogrel, prasugrel reduced the risk of the composite end point of death from CV cause, MI or stroke by 19% (HR 0.81; P<0.001) but increased the risk of non-CABG-related major bleeding by 32% (HR 1.32; P=0.03). There was no difference in mortality. Post-hoc analyses indicated that the elderly (age ≥75 years) and those with a body weight <60 kg did not gain a net clinical benefit when you include major bleeding in the primary end point. Furthermore, the small number of patients with a history of stroke or transient ischemic attack (TIA) had an increased risk of adverse outcomes from prasugrel use. Conversely, following angioplasty, prasugrel had a particularly important net advantage over clopidogrel in patients with diabetes (Wiviott et al. Circulation 2008;118:1626-36). These data must be used to provide guidance for candidate selection.
In the PLATO trial, all individuals admitted to hospital with ACS were randomized regardless of planned procedure or pre-hospital antiplatelet treatment to a ticagrelor loading dose of 180 mg followed by a 90-mg twice daily maintenance dose or a clopidogrel loading dose of 300 or 600 mg followed by a 75-mg daily maintenance dose. Both groups received ASA. Approximately 37% of the 18,624 patients randomized had STEMI and the remaining had NSTEMI. Relative to clopidogrel, ticagrelor reduced the risk of the composite end point of death from vascular causes, MI or stroke by 16% (HR 0.84; P<0.001). The difference in total major bleeding (11.6% vs. 11.2%; P=0.43) did not reach statistical significance, but non-CABG bleeding was significantly increased by 19% (4.5% vs. 3.8%; P=0.03). Noteworthy to antiplatelet trials, ticagrelor was associated with a 22% reduction (HR 0.78; nominal P<0.001) in all-cause mortality. The benefit on the primary end point was not significant in North America, possibly due to the higher dose of ASA used in the US (Mahaffey et al. Circulation 2011;124(5):544-54). In chronic renal failure, ticagrelor demonstrated a particularly large advantage on mortality over clopidogrel and though the P-value for interaction did not reach significance (P=0.16), these data are reassuring for this vulnerable population (James et al. Circulation 2010;122:1056-67).
New Data Translated into Clinical Practice
While all ACS patients should be initiated on ASA immediately, choice of the second antiplatelet agent is defined by the diagnosis, the planned strategies for intervention and specific patient characteristics. Several large organizations have altered ACS antiplatelet guidelines on the basis of the PLATO and TRITON-TIMI 38 trials, but algorithms at the regional or hospital level are appropriate because of differences in ACS care.
As a first choice in NSTEMI, patients can be started on ticagrelor or switched from clopidogrel immediately in the ER. While ticagrelor is the better option in a dual antiplatelet strategy with ASA, there are important exceptions according to data derived from PLATO. These include patients on an anticoagulant, patients who have had a prior intracranial hemorrhage, patients who take a potent modulator of cytochrome 3A4/5 (Table 1), and patients expected to be non-compliant to medication, particularly if an invasive strategy is contemplated. Ticagrelor should not be employed with high-dose ASA. In those not candidates for ticagrelor, clopidogrel remains the preferred partner with ASA. Prasugrel is limited to patients who underwent a coronary angioplasty or may be given to those directed emergently in primary PCI.
Table 1.
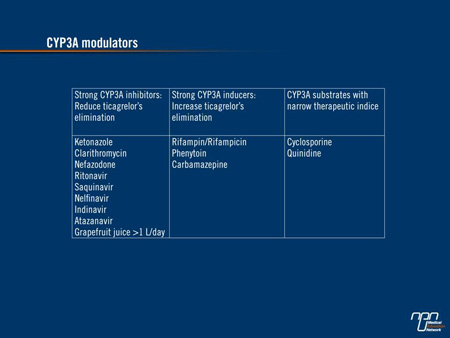
In STEMI, the data require more interpretation, but the newer agents are attractive. One reason is that both prasugrel and ticagrelor permit a faster antiplatelet effect than even high doses of clopidogrel. However, the 600 mg dose has never been demonstrated superior to 300 mg for patietns scheduled for a primary PCI in any large study. Overall, CURRENT-OASIS 7 was a negative study even if the P-value for cardiac events was marginally significant for the higher dose in the non-predictable subgroup of STEMI and NSTEMI patients who had angioplasty (HR 0.86; P=0.039). This advantage was achieved at a cost of an increase in major bleeding (HR 1.41; P=0.009) (Mehta et al. Lancet 2010;1233-43).
We need to rely on substudy analyses to guide antiplatelet therapy in STEMI. But there were not enough STEMI patients in either PLATO or TRITON directed to primary PCI in <12 hours of symptoms to draw firm conclusions. Yet the benefits in this subgroup did appear to be similar to that observed in the whole study population. In TRITON, this included a reduction in the secondary end point of stent thrombosis. As a result, we have elected at my institution to administer prasugrel 60 mg in the ER for STEMI patients <75 years old and >60 kg without a history of stroke or TIA. For patient in cardiogenic shock or those who do not fulfill criteria for prasugrel, we encourage the use of ticagrelor. We believe that ticagrelor is more easily absorbed in cardiogenic shock (Orban et al. Platelets 2012;23(5):395-8) and its reversibility makes it a choice agent if a surgery is anticipated. Clopidogrel is restricted to patients with active bleeding, history of intracranial bleeding or on anticoagulants.
Relevance of New Algorithm to Regional Centres
The objective evidence that newer antiplatelet therapies can improve the outcome in ACS patients informs but does not dictate adjustments in patient care. Each patient is different and does not necessarily fit the criteria used in the TRITON and PLATO studies. Due to substantial regional disparities in the care of ACS driven largely by variability in resources, such as the proximity of rapid response teams and differences in transfer intervals to catheterization laboratories, treatment guidelines must be adjusted for relevance to current practice. For example, the improvement in outcome associated with prasugrel in STEMI patients scheduled for PCI is based on the availability of PCI. At regional centres, treatment algorithms must be adjusted for these variables. A large number of medically treated patients never go to the catheterization laboratory. These high-risk patients have a large mortality reduction when ticagrelor is used. All opportunities to improve outcome with more effective antiplatelet regimens should not be overlooked.
Too often, fear of a complication overcomes the established knowledge of their efficacy advantage. It is impossible to document a stent thrombosis that has been avoided with a more potent antiplatelet, but there will be suspicion that antiplatelet therapies were involved in a bleeding event. Nevertheless, for the benefit of the patient, we should follow an evidence-based practice. With physicians less familiar with the newer antiplatelet agents, a local protocol may be useful to support practice and remind indications of these new therapies.
There are compelling data to conclude that implementation of more modern strategies for appropriate candidates will improve ACS outcomes including a reduction in mortality. The implementation and adherence to treatment guidelines in the management of ACS have been associated with statistically significant improvements in outcome. In an observational analysis that included 350 academic and non-academic centres, a stepwise 10% reduction in in-hospital mortality rates was associated with each 10% increase in adherence to evidence-based guidelines (Peterson et al. JAMA 2006;295(16):1912-20).
Conclusion
The first-line antiplatelet strategies in ACS patients have been revised. The newer agents ticagrelor and prasugrel provide an important opportunity to improve outcome relative to clopidogrel when combined with ASA. In NSTEMI patients, the advantage of ticagrelor over clopidogrel in appropriately selected patients includes a mortality reduction. Post-angioplasty, patients particularly at high risk of stent thrombosis (such as those with diabetes, chronic renal failure or complex multivessel disease) should be switched if they are not already on a newer antiplatelet agent. In STEMI, the data are less robust, but common sense and guidelines support the use of prasugrel or ticagrelor over the previous clopidogrel standard. Finally, before large scale studies compare directly prasugrel to ticagrelor, it will remain difficult to claim the superiority of one agent over the other. Meanwhile, we must work with available data and use these agents instead of clopidogrel where they have shown superiority in the best interest of ours patients.
Questions & Answers
Q: What is your perspective on the benefit-to-risk ratio that the newer antiplatelet agents offer for reducing the risk of thrombosis within an acceptable rate of bleeding?
A: Both agents are associated with a reduction in stent thrombosis, but they also increase non-CABG bleeding. As an interventional cardiologist, I feel that a stent thrombosis is a more devastating event than a major bleed. Numerically, the benefit in stent thrombosis relative to non-CABG major bleeding was favourable in TRITON and almost equivalent in PLATO, but bleeding definitions were different in the 2 studies. Some patients may be more prone to stent thrombosis. These are patients with diabetes, chronic renal failure, neoplasia, cardiac failure or those with previous stent thrombosis. For others, as those with stent in the left main or those stented on the last functional vessel, we cannot afford a stent thrombosis. If they are not on a new antiplatelet agent, they should be switched.
Q: The studies that led to changes in the guidelines compared therapies in different populations. What insights can you offer on why it was important to prove superiority of prasugrel or ticagrelor over clopidogrel in different ACS groups (STEMI, NSTEMI, unstable angina, etc)?
A: We have a good example with the result of the TRILOGY study, which failed to prove superiority of prasugrel over clopidogrel in ACS medically treated. For this reason, ticagrelor remains the only alternative to clopidogrel when a non-invasive strategy is chosen. We hope the result of the ongoing PEGASUS study will support this affirmation. STEMI is a different situation where time is very important, specific concomitant therapies are often used (fibrinolysis, bivaluridin, GPIIb/IIIa inhibitor) and the probability of immediate or early angioplasty is very high. The benefit:risk ratio is expected to be different. For the same reason, we can’t extrapolate the results of the studies of newer antiplatelet agents in ACS to elective angioplasty in stable patients who have not yet been studied.
Q: What is your point of view on the possible side effects associated with the newer agents vs the opportunity to improve outcomes?
A: Except for increased bleeding, side effects with prasugrel are the same as those with clopidogrel. But the increase in bleeding relative to the benefit is around the same as the one we had accepted a decade ago, following the CURE study, when clopidogrel was included in treatment of ACS with a Class 1 indication. For patients who go in CABG, bleeding is more worrisome and a delay of at least 7 days must be observed when possible. The retrospective analysis of patients undergoing CABG in TRITON, showed an increase of bleeding and transfusions in the prasugrel group but the mortality rate was significantly lower than patients in the clopidogrel group (Smith et al. JACC 2012;60(5):388-96). Ticagrelor is a first agent of the new cyclo-pentyl-triazolo-pyrimidine class. Being a totally different molecule makes it also the alternative of choice when we suspect an allergic rash with a thienopyridine, as clopidogrel, prasugrel or ticlopidine. However, there are new side effects of which clinicians must be aware. Dyspnea and sinusal pauses can be observed at the introduction of the medication and usually vanish after a few days or in some cases a few weeks. These effects seem to be related to the similarity of the ticagrelor’s molecular structure to adenosine. In close monitoring of the PLATO study, only 0.9% of patients stopped the medication because of shortness of breath, but compared to clopidogrel (0.1%) that was significant. In real life we should be attentive. Compliance is an important issue for a medication that should be taken twice a day and could have side effects. Non-CABG bleeding is increased with ticagrelor, but not CABG related bleeding with the soft definition of major bleeding used in PLATO. Nevertheless, the ticagrelor monograph recommends stopping the medication for 5 days before a planned surgery. Ticagrelor is not an agent that you can easily reverse with platelet transfusion. But the reversible fixation at the P2Y12 receptor and the short half-life (7-8 hours) makes the level of platelet inhibition relatively low 48 hours after the last dose. In PLATO, the subgroup of patients undergoing CABG within 7 days of the last study drug intake, ticagrelor was also associated with a significant reduction in total mortality (Held et al. JACC 2011;57(6):673-84).
Q: The algorithm introduces some decision points not previously required when all patients were treated with clopidogrel plus ASA. What action needs to be taken to improve outcome?
A: Every centre should be encouraged to develop protocols to help physicians adopt a practice in accordance with new evidence. By giving specific indications, these protocols support the choice of using appropriately new agents. By including contraindications, the protocol can reassure physicians they are not overlooking something important. The role of protocols is to support decision making and should never be coercive nor too rigid, because they do not replace guidelines, common sense and the evolving literature. Education is still an important way to translate guidelines into practice and to motivate use of established protocols.