Reports
AIM-HIGH Study: Evaluating Results in the Context of the HDL-C Hypothesis
This report is based on medical evidence presented at sanctioned medical congress, from peer reviewed literature or opinion provided by a qualified healthcare practitioner. The consumption of the information contained within this report is intended for qualified Canadian healthcare practitioners only.
PHYSICIAN PERSPECTIVE - American Heart Association (AHA) Scientific Sessions 2011
Orlando, Florida / November 12-16, 2011
Guest Editor:
Ruth McPherson, MD, FRCPC
Director, Atherogenomics Laboratory
and Lipid Clinic
Professor of Medicine and Biochemistry
University of Ottawa Heart Institute
Ottawa, Ontario
Introduction
There is a compelling rationale for targeting low levels of high-density lipoprotein cholesterol (HDL-C) to reduce cardiovascular (CV) risk. Low plasma concentrations of HDL-C have long been recognized as one of the strongest predictors of future CV events.1 Raising HDL-C has been particularly attractive for residual risk reduction in patients who have reached target levels of low-density lipoprotein cholesterol (LDL-C).2 The recently published AIM-HIGH trial, conducted with extended-release niacin, had the potential to confirm that treating HDL-C reduces risk beyond that provided by LDL-C-lowering therapy.3 The failure of the study to show this benefit is a lesson in the challenges of definitively proving the HDL-C hypothesis. This hypothesis, which simply states that HDL-C is a treatable CV risk factor, was not adequately addressed by AIM-HIGH, and the results of AIM-HIGH do not rule out a potentially important role for niacin in CV risk management.
A long series of large multicentre trials have demonstrated that reductions in low-density lipoprotein cholesterol (LDL-C) with statins provide major protection against the risk of cardiovascular (CV) events for patients in the primary or secondary prevention categories. The clinical benefit is amply supported by the favourable impact of LDL-C reduction on atherosclerosis regression and vascular function.4
It has long been hoped that high-density lipoprotein cholesterol (HDL-C) is an analogous treatment target. In particular, the tight correlation between HDL-C and risk of CV disease is consistent with its known activity in reverse cholesterol transport, which promotes efflux of cholesterol from peripheral tissues.5 While the inverse relationship of HDL-C and CV risk is well documented in epidemiologic studies, the missing link has been a definitive demonstration that treatments which raise HDL-C will lower CV risk.
The lack of definitive evidence should not be confused with lack of evidence. In the Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial, an 11% reduction in coronary heart disease was observed with every 5 mg/dL (0.13 mmol/L) increase in HDL-C achieved with gemfibrozil.6 In the Coronary Drug Project, niacin was not only associated with a reduction in risk of CV events—including a 27% reduction (P=0.004) in recurrent non-fatal myocardial infarction (MI)—but also an 11% (P=0.0004) all-cause mortality reduction.7 In a meta-analysis of 11 randomized studies, which also documented regression of atherosclerosis, niacin was associated with a 25% reduction (P<0.0001) in major coronary events, a 26% reduction (P=0.007) in stroke and a 27% reduction (P<0.0001) in CV events of any kind relative to controls.8
On the basis of these studies and other evidence, therapies that raise HDL-C are widely used to reduce CV risk, particularly in individuals who have not reached LDL-C targets and have depressed HDL-C levels (<1.0 mmol/L in males, <1.3 mmol/L in females). This is consistent with current guidelines. The guidelines employ the word “consider” HDL-C-raising therapies in such patients, rather than a stronger term, while awaiting definitive trials.
Several studies, including AIM-HIGH, have attempted to confirm the HDL-C hypothesis much in the way that the statin trials confirmed the LDL-C hypothesis. The largest of these, ILLUMINATE, randomized 15,067 patients at high CV risk to the CETP inhibitor torcetrapib plus atorvastatin or atorvastatin alone.9 The trial was halted prematurely because of higher rates of mortality in the torcetrapib group, although subsequent analyses have attributed the increased risk to off-target effects, particularly effects on the renin angiotensin system and elevated blood pressure.10 Other CETP inhibitors do not appear to share these off-target properties, and 2 major phase III trials are now underway with newer agents from this class.
AIM-HIGH Design
The goal of AIM-HIGH was to demonstrate a benefit from extended-release niacin when added to simvastatin in patients with established CV disease and low levels of HDL-C. The primary end point was a composite of death from coronary heart disease, nonfatal MI, ischemic stroke, hospitalization for an acute coronary syndrome (ACS), or symptom-driven coronary or cerebral revascularization. The 3414 patients who participated were randomized to receive niacin 1500 to 2000 mg/day or matching placebo. All patients received simvastatin 40 to 80 mg plus ezetimibe 10 mg, if needed to maintain LDL-C between 1 and 2 mmol/L.
The trial was stopped prematurely because of a lack of efficacy. The event rates for those who did or did not receive niacin at the time the study was halted were almost identical (16.4% vs. 16.2%). The early discontinuation of the study was hastened by a slightly higher rate of stroke in the niacin arm (1.6% vs. 0.9%) at the time of the interim analysis. This has not been observed in previous niacin studies and there was no difference in rate of stroke in AIM-HIGH, when the final data analysis was completed, with on-treatment stroke events being similar in the 2 groups (21 in niacin arm; 18 in placebo arm). The authors concluded that in patients with CV disease and LDL-C <1.8 mmol/L, there was no incremental benefit from the addition of niacin even though HDL-C levels (median 0.91 mmol/L at entry vs.
1.08 mmol/L at end) were significantly raised.
AIM-HIGH Design: Lessons
The AIM-HIGH data have provided insight into the difficulties of proving the HDL-C hypothesis. While there has been controversy about aspects of the AIM-HIGH design, e.g. the decision to provide a small dose of niacin to the placebo group in order to maintain blinding, a significant issue was the small difference in end-of-study HDL-C: while HDL-C increased significantly on niacin, levels increased unexpectedly in the placebo arm so that there was less than a 10% difference at study end (1.08 vs. 0.98 mmol/L). Although AIM-HIGH was intended to have an 85% power to detect a 25% reduction in CV events, the study, which was terminated at 550 rather than the planned 800 events, had sufficient power to detect only a 10% difference in events by the end of study based on the observed HDL-C differences between the two groups. Moreover, nearly 25% of patients discontinued therapy during the trial, further weakening the potential for discrimination.
It is notable that during the trial, 28.2% of the placebo group vs. only 17.7% of the niacin group were on a 60-mg
or greater dose of simvastatin. Moreover, more than twice as many placebo patients were also taking ezetimibe
(21.5% vs. 9.5%; P<0.001). Despite the greater use of lipid- lowering therapies in the placebo arm, not only were the HDL-C increases higher, as would be predicted with niacin, the median levels of LDL-C (1.6 vs. 1.73 mmol/L) and triglycerides (3.1 vs. 3.9 mmol/L) at the end of 3 years on therapy were lower in the group receiving niacin.
The goal of this study was to demonstrate a reduction in residual risk among patients who were already near LDL-C targets. Almost all (94%) the patients had been on statin therapies prior to starting niacin, and 20% had previously taken niacin. This type of aggressive management of hyperlipidemia prior to study entry may have limited the ability to demonstrate additional risk reduction by raising HDL-C. Long-term stabilization of atherosclerotic plaques would be expected to reduce potential benefits of increasing HDL-C and improving reverse cholesterol transport. Indeed, the event rate overall in AIM-HIGH was lower than projected in the design.
It should be noted as well that the study was terminated prematurely and the benefits of HDL-C-raising may accrue over a longer time period as compared to that observed for LDL-C-lowering therapies. The fact that a non-significant increase in the rate of strokes was a contributing factor to an early termination is disconcerting because of the problems introduced when non-specified events influence decisions about conduct of objective studies. Previous studies, including the Coronary Drug Project, and a recent meta-analysis of niacin studies demonstrated that niacin treatment is associated with protection from stroke, and there is no plausible biologic explanation for an increased risk of stroke from niacin. In fact, the on-treatment stroke rates did not differ significantly in the final analysis.
Overal, AIM-HIGH does not support the use of niacin for risk reduction in the study population evaluated. However, it does not refute the potential for benefit for those patients who fail to reach LDL-C targets on statin therapy or for those patients who are statin-intolerant. It must be remembered that a significant percentage of high-risk patients do not achieve LDL-C levels of ≤2.0 mmol/L on statin therapy alone.
Summary
Several studies with the potential to confirm the HDL-C hypothesis have failed to do so for a variety of reasons. While HDL-C may prove to be a more complex molecule than LDL-C in regard to its relationship to CV risk and its potential as a target for CV risk reduction, the HDL-C hypothesis remains to be properly evaluated. HDL-C is a powerful marker of CV risk and a potential target for therapy. Ongoing clinical trials including HPS2-THRIVE may provide a more definitive answer.
Questions & Answers
The following section is based on discussions during the recent AHA scientific sessions with Dr. Ruth McPherson, University of Ottawa Heart Institute; Dr. Jean C. Grégoire, Institut de cardiologie de Montréal; and Dr. Todd J. Anderson, Department of Cardiac Sciences, University of Calgary.
Q: The discussants invited by the AHA to critique the AIM-HIGH trial suggested that there were several factors in the trial design that precluded a positive result. Do you agree?
Dr. McPherson: AIM-HIGH was an event-driven trial designed to have an 85% power to detect a 25% reduction in CV events. It was calculated based on the anticipated differences in HDL-C and LDL-C that a sample size of 3400 participants followed for 2.5 to 7 years would generate the required 800 primary events. The expected differences in lipids were not achieved in part because of study design. In terms of HDL-C, treatment with niacin increased the level of HDL-C by 25% to an on-treatment level of 1.08 mmol/L.
However, for unknown reasons, the level of HDL-C also increased substantially in the placebo group to an on-treatment level of 0.98 mmol/L. Consequently, the on-treatment difference in HDL-C between the two groups was only 0.10 mmol/L. In terms of LDL-C, the group randomized to statin plus placebo received significantly higher doses of simvastatin and was prescribed additional ezetimibe twice as often as those randomized to statin plus niacin. Thus, the median on-treatment levels of LDL-C were 1.60 and 1.73 mmol/L in the niacin and placebo arms, respectively, a difference of only 0.13 mmol/L. Based on the observed differences in lipids, a very small benefit (~12%) in terms of decreased CV disease events on niacin would be predicted. Importantly, the study was not designed to answer the important clinical question, “What is the benefit of the addition of niacin to a statin in patients not achieving optimal LDL-C goals on a statin alone?”
Dr. Grégoire: Several reasons could explain why the results of the AIM-HIGH trial were neutral. The number of patients (N=3400) was calculated on the basis that the cohort would provide an 85% power to detect a 25% reduction in CV events over a median follow-up of 4.6 years. This calculation assumed that 800 events would take place over this period; however, the event rate was far lower (550 events), reducing the power to detect by 12.5%. It therefore appears that the cohort size and duration of follow-up were far from adequate to show a clinical benefit with niacin. In terms of HDL-C, the difference between the 2 groups was less than expected, i.e. only 0.10 mmol/L, because of an unforeseen and hard-to-explain HDL-C increase in the placebo group. In addition, 93% of patients were already on a statin at randomization. The median end-of-study LDL-C was 1.60 mmol/L
in the niacin group vs. 1.73 mmol/L in the placebo group, which represents a difference of 0.13 mmol/L. This very small difference between the 2 groups and the impact of a long-term statin therapy helped reduce the number of CV events in this optimally-treated patient cohort.
Dr. Anderson: The AIM-HIGH study was strongly criticized by firm believers in the HDL-C hypothesis, but the trial design was not a black-or-white test of HDL-C as a targetable risk factor. Rather, the question asked by the study was whether adding niacin in patients with a high-risk phenotype who are already on aggressive LDL-lowering therapy reduces risk of CV events. It is important to recognize that 80% of these patients had the metabolic syndrome or diabetes. In this type of patient, a reasonable dose of niacin did not provide a further reduction in risk over already low LDL-C levels. I do not agree completely that the trial design was flawed for the question that was being asked.
Q: When the study was stopped early, an imbalance in the rate of stroke between the treatments groups was noted based on the interim data. Is this a concern based on the final data?
Dr. McPherson: The difference in stroke was not significant. Importantly, 8 strokes in the niacin group occurred 2 months to 4 years after discontinuation of niacin treatment. Stroke incidence on treatment was similar in both groups.
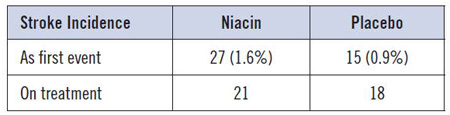
Dr. Grégoire: Surprisingly, 27 (1.6%) ischemic strokes occurred as first events in the niacin group vs. 15 (0.9%) in the placebo group. Of these patients, a total of 8 from the niacin group had an ischemic stroke 2 months to 4 years after discontinuing their treatment. Considering the difference between the 2 groups is not statistically significant, we should be reassured regarding the use of niacin.
Dr. Anderson: The signal for stroke observed in the interim analysis was not the main driver for the decision to terminate the trial early. It was terminated for futility. The difference in the rate of stroke was barely statistically significant at the time it was decided to terminate the study and was only a trend in the final analysis. I am not concerned that niacin increases the risk of stroke on the basis of these data. There is no good mechanistic reason to anticipate an increased risk of stroke, and none of the previous studies have associated niacin with an increased stroke risk. Rather, there are some studies suggesting niacin provides protection against stroke.
Q: The HDL-C hypothesis is very strongly supported by population-based data but has not yet been proven in a prospective study. Do you think that AIM-HIGH had any value for exploring the HDL-C hypothesis?
Dr. McPherson: A larger difference in HDL-C between the 2 groups may well have been required to answer the question as to whether raising HDL-C with niacin is of clinical benefit. It may also be true that increasing HDL-C concentrations is less important for patients for whom tight LDL-C control has been achieved.
Dr. Grégoire: The HDL-C hypothesis was not tested adequately in the AIM-HIGH trial considering the trial design and the small HDL-C difference between the active and placebo groups. Furthermore, in patients with LDL-C already on target, the benefit of an HDL-C increase is more difficult to prove.
Dr. Anderson: There are a number of potential explanations to explain why a 15% relative increase in HDL-C did not provide protection against CV events in AIM-HIGH. Of these, one of the most provocative is the potential effect of long-term statin therapy. Most of these patients had been on statins long before entering the study, and it may be that these stabilized the plaques to such a degree that raising HDL-C had no added benefit, particularly in regard to their role in efflux of lipids from the vascular wall. Although it is important to consider the possibility of a disconnect between the epidemiologic evidence that would predict benefit from HDL-C-raising and CV protection—which we have seen before in regard to homocysteine—this study has not substantially weakened the HDL-C hypothesis. We may need agents that more specifically raise HDL-C and offer them to patients with vulnerable plaques in which higher HDL-C levels might provide the most benefit.
Q: What is the impact of the AIM-HIGH study on clinical practice?
Dr. McPherson: Perhaps relatively little, since the majority of niacin is prescribed for patients who have not achieved LDL-C goals. Even today, as shown in the recent LTAP-2 survey, the mean LDL-C achieved for high-risk patients is 2.35 ± 0.85 mmol/L. Thus the majority of high-risk patients remain above target.
Dr. Grégoire: The AIM-HIGH trial will probably have a minor impact on present clinical practice. However, many patients do not reach targets with a single statin or do not tolerate statins. In these patients, the addition of niacin alone or in combination is indicated to lower LDL-C to target.
Dr. Anderson: The results of AIM-HIGH have not had an effect on my practice. Although I have not been prescribing niacin widely, I do use it in patients who do not tolerate statins or have familial disease that predicts a high risk of CV events even with optimal LDL-C lowering. The data from AIM-HIGH do not provide any information about the potential benefit from niacin for these indications. Although we do need more evidence that niacin is effective in these patient groups, there are data to support these uses and we have limited alternatives.
Q: How do you currently manage patients with residual risk after optimal lowering of LDL-C with elevated triglycerides or low HDL-C?
Dr. McPherson: Start with a more potent statin and titrate to highest dose tolerated. If an LDL-C of <2.0 mmol/L is not achieved, consider the addition of a second agent. Niacin is a second agent of choice if triglycerides are also elevated.
Dr. Grégoire: First of all, statin therapy must be initiated and the dose must be titrated up to the maximum tolerable dose. If the LDL-C target is still not reached, a second agent should be considered. Niacin remains an adequate choice in patients with low HDL-C and/or high triglycerides. A drastic change in lifestyle should also be considered in all patients with dyslipidemia.
Dr. Anderson: If a patient still has progressive disease and has optimally lowered LDL-C, it still makes sense to consider niacin. We certainly need more data and perhaps better therapies for raising HDL-C in these patients, but
AIM-HIGH did not necessarily evaluate patients with progressive disease. Again, the AIM-HIGH study demonstrated that adding niacin to high-risk patients on long-term statin therapy, with very low levels of LDL-C, did not provide a significant risk reduction over the period that patients were followed, but the data are not applicable outside of this study population.
References
1. Gordon et al. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am J Med 1977;62(5):707-14.
2. Barter et al. HDL cholesterol, very low levels of LDL cholesterol, and cardiovascular events. N Engl J Med 2007;357(13):1301-10.
3. Boden et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med 2011;365(24):2255-67.
4. Nicholls et al. Effect of two intensive statin regimens on progression of coronary disease. N Engl J Med 2011;365(22):2078-87.
5. Lewis GF, Rader DJ. New insights into the regulation of HDL metabolism and reverse cholesterol transport. Circ Res 2005;96(12):1221-32.
6. Rubins et al. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group. N Engl J Med 1999;341(6):410-8.
7. Canner et al. Fifteen year mortality in Coronary Drug Project patients: long-term benefit with niacin. J Am Coll Cardiol 1986;8(6):1245-55.
8. Bruckert et al. Meta-analysis of the effect of nicotinic acid alone or in combination on cardiovascular events and atherosclerosis. Atherosclerosis 2010;210(2):353-61.
9. Barter et al. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med 2007;357(21):2109-22.
10. Clerc et al. Mechanisms underlying off-target effects of the cholesteryl ester transfer protein inhibitor torcetrapib involve L-type calcium channels. J Hypertens 2010;28(8):1676-86.