Reports
Malnutrition: A Serious Condition Requiring Early Evidence-based Nutritional Intervention
This report is based on medical evidence presented at sanctioned medical congress, from peer reviewed literature or opinion provided by a qualified healthcare practitioner. The consumption of the information contained within this report is intended for qualified Canadian healthcare practitioners only.
RESOURCE LINE - Nutrition
December 2011
Among key components of disease management, evidence-based data indicate that proper nutritional intervention can improve outcomes and in many cases lower costs of care. Routine early nutritional screening permits intervention to prevent or reverse malnutrition and provide a baseline value for following and addressing adverse subsequent changes in nutritional status. However, in hospitalized and chronically ill patients, there is an absence of systematic processes to detect protein energy malnutrition (PEM) leading to preventable morbidity and mortality. PEM is a distinct form of malnutrition characterized by loss of protein stores critical to processes that mediate recovery. In individuals living in the community, particularly the elderly, PEM can be a significant contributor to frailty and accelerated deterioration in health status. As such, screening for PEM is relevant to individuals in a variety of settings including acute and long-term care facilities. Therefore, clinical practice needs to keep pace with the science of malnutrition detection and management.
Chief Medical Editor: Dr. Léna Coïc, Montréal, Quebec
Malnutrition Leading to Loss of Lean Body Mass
Malnutrition, sometimes unrelated to total calorie consumption, occurs when insufficient intake of nutrients leads to adverse changes in normal physiology. In a broad sense, the term malnutrition includes all forms of inappropriate nutrition, including undernutrition or overnutrition. In the absence of adequate nutritional intake for protein synthesis, the tissue and organ demand for amino acids leads to depletion of endogenous protein stores, diminishing muscle mass and initiating a process that can lead to cachexia, a weakness and
wasting of the body in the case of severe chronic illness.
In industrialized countries such as Canada, the complications of malnutrition in patients with chronic diseases are primarily the result of loss of lean body mass (LBM). LBM constitutes about 75% of body weight and refers to all tissues, such as muscle and bone, but excludes fat. Age-related loss of muscle mass or sarcopenia1 begins in middle age and accelerates more quickly at around age 60.2
Malnourished patients suffer from nutrient deficiencies important to specific organ functions. Protein metabolism is an essential component of the healing process. Protein energy malnutrition (PEM) occurs when changes in metabolism reduce the synthesis of new proteins in relation to protein breakdown, thus creating an imbalance. PEM leads to loss of LBM or skeletal muscle, and can therefore cause sarcopenia.
In a health care setting, it is important to recognize that malnutrition in its early stages may not be immediately noticeable through patient appearance. A low body mass index (BMI), a relatively insensitive marker for skeletal muscle mass, has been associated with an increased risk of mortality in elderly patients.3 Risk rises precipitously when BMI decreases to a level of ≤21 kg/m2. In a multinational
study of more than 5000 adult hospitalized patients without age restrictions, the mortality rate reached 12% in those with nutritional deficits during screening vs. 1% in those without (P<0.001)4. Although low body weight has also been found to be an independent predictor of increased mortality risk in nursing home patients,5 failure to screen for malnutrition independently of low body weight can lead to undiagnosed cases of malnourished patients.
Balanced nutritional intake that meets individual requirements typically replenishes a broad spectrum of essential nutrients, including protein and vitamins. In fact, protection and restoration of LBM is one of the most urgent clinical issues for chronic disease patients with PEM.
Malnutrition Rates and Clinical Implications
While insufficient nutrient intake may contribute to PEM, the stress and increased metabolic demands of specific diseases vary, explaining why some disease states are more closely associated with PEM than others. In an overview of prognostic importance, disease-related malnutrition was associated with increased mortality and morbidity from both acute and chronic conditions, increased length of hospital stay and higher treatment costs.6 For example, some studies estimate that about 25% of hospitalized patients have or
are at risk of PEM;7 the rate appears much higher in cancer patients, where rates can exceed 50% for certain types of cancer.8 Chronic obstructive pulmonary disease (COPD), heart failure and gastrointestinal (GI) diseases are also among a list of conditions that pose an elevated risk of PEM.9-12 Over a 6-month period in COPD patients at risk for malnutrition, mortality rates were almost threefold greater
(16.3% vs. 5.5%; P=0.023) than in those who were not.13 In patients with GI disease, malnutrition is associated with reduced muscle function and impaired quality of life (QOL).14 Patients who have had GI problems and who have undergone GI surgery constitute an important group at risk for malnutrition.
Among the elderly, disease-related malnutrition has been associated with a threefold increase in mortality.15 Even in the absence of a chronic disease, the elderly have a high risk of malnutrition and the rate of PEM increases across all disease states. While the degree of skeletal muscle mass loss in any specific individual appears to be multifactorial, it is estimated that up to one-third of aging patients may develop cachexia prior to death.16 Among the elderly, rates are not only high in acute care facilities17 but also in long-term care facilities,
nursing homes and the community setting.18 The reasons are multifactorial, including reduced appetite and impaired mobility that can restrict abilities to purchase food and prepare meals, but the rising proportion of elderly patients makes this an increasingly important public health concern.
As predicted by its fundamental role in essentially all physiologic processes, loss of LBM is also associated with a wide variety of morbidities. A large amount of experimental and clinical evidence has demonstrated an association between PEM and impaired wound healing,19 which may be relevant to decubitus ulcers, risk of infection and length of hospital stay. Rates of nosocomial infections have been found to be approximately 3 times more frequent in malnourished hospitalized patients than in those who are not.20 These clinical
issues occur independently of reduced muscle strength, which can adversely affect functional status21 including self-care, increased risk of accidents22 and diminished QoL at least partly driven by loss of independence.23
Evidence-based Intervention
The diagnosis and treatment of PEM in patients with an acute or chronic disease deserves the same type of rigorous and systematic approach as other concomitant problems contributing to poor clinical outcomes. The misperception that correcting nutritional deficits is a secondary consideration to disease control overlooks the important contribution that malnutrition is making to the underlying disease process. The ability of oral nutritional supplements (ONS) to reverse the risks of depleted protein-energy stores and improve
clinical outcomes is likely to be relevant in many individuals with PEM.
Mortality reductions provide the most profound evidence of clinical benefits. From this perspective, there are many studies associating ONS with improved outcomes. A meta-analysis of more than 20 randomized, controlled trials with ONS prepared for the UK National Institute for Health and Clinical Excellence (NICE) nutritional guidelines demonstrated that the hazard ratio (HR) for death was reduced by 18% (HR 0.82; 95% CI, 0.68-0.98; P<0.01) for those receiving active treatment relative to controls.24 In another meta-analysis of
11 studies, the risk reduction was 39% (HR 0.61; 95% CI, 0.48-0.78; P<0.001).25 In a third meta-analysis that was limited to patients who had documented malnourishment at baseline, the relative risk reduction for mortality in 21 trials was 28% (HR 0.72; 95% CI, 0.55-0.94; P<0.01).26 Although not all trials have demonstrated a statistically significant reduction, which is perhaps explained by different ONS formulations and different populations studied, there is a preponderance of evidence for a measurable mortality reduction in diverse populations with chronic diseases.
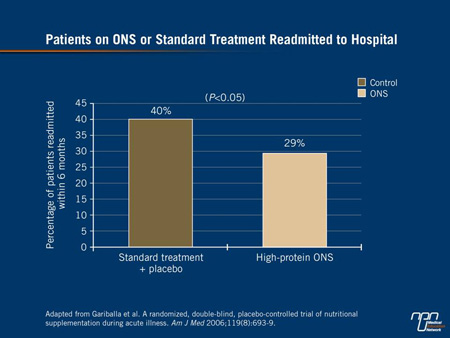
Regarding improvement in health status, the reduction in risk of hospital readmission is also considered a powerful indicator. In one double-blind, randomized study of 445 hospitalized patients with a broad representation of admitting diagnoses, the reductions in length of hospital stay and mortality on ONS vs. usual care did not reach significance. However, the reduction in readmission rates within 6 months did (29% vs. 40%; P<0.05)27 (Figure 1). In a second randomized study in which ONS were associated with improvement in functional measures such as grip strength, as well as an improvement in QoL, readmission rates were reduced over 3 months by 50%
(P=0.041).12 Numerous other measured outcomes, including a reduction in the risk of overall complication rates and in decubitus ulcers specifically,26,28,29 have also been associated with ONS relative to a control form of management, often usual care without nutritional supplementation.
Figure 1.
Cost analyses conducted in the course of ONS studies also provide support for this intervention. For example, on the basis of prevention of decubitus ulcers, ONS have been shown to provide cost savings when ulcers are severe (stage ≥III). On a broader spectrum, cost savings from ONS vs. no nutritional supplementation have been calculated from projections as well as actual cost savings stemming from a reduction in hospital stay.30,31 Such cost savings are highly dependent on patient selection criteria, but a reduction in costs has been observed in the community setting when ONS were employed in high-risk elderly patients to prevent hospital admissions and
reduce health care use.32 Even on the basis of quality-adjusted life-years (QALYs) to calculate improvement in QoL, ONS were found to be cost-effective by normative standards in a controlled trial involving patients with benign GI disease.33
Practical Considerations
The consequences of PEM cannot be prevented if those at risk are not first identified. The existing evidence that treatment
of malnutrition with ONS reduces health risks, including mortality, provides the evidence-based platform for developing systematic approaches to diagnosis. At hospitals surveyed in Europe, approximately 40% had no automatic nutritional screening program for patients at admission or subsequent follow-up.34 In Canada, no survey results from hospitals have been published yet, but it is known that such approaches are not uniform. Effective screening programs exist and require a uniformly applied protocol in order to improve clinical care.
Helpful nutritional screening tools include the Nutritional Risk Screening tool (NRS 2002) and the Malnutrition Universal Screening Tool (MUST). MUST includes calculations for the risk associated with the underlying disease state as well as factors such as recent weight loss and baseline BMI.35 On the other hand, NRS-2002 contains the nutritional components of MUST, and in addition, a grading
of severity of disease as a reflection of increased nutritional requirements.36 Other tools are available but the goal remains the same: to evaluate nutritional status as a routine part of initial clinical assessment with strategies for subsequent reevaluation over the course of care. Such strategies are not only appropriate in acute care settings but also in long-term care facilities and even in the outpatient setting, particularly in elderly populations among which the risk of malnutrition is greatest. Nutritional interventions can then be tailored based on screening results.
In patients who meet criteria of PEM, nutritional supplementation is the first-line therapy. For patients who have the ability to eat, ONS used as sole source nutrition—or more commonly in conjunction with meals—can provide the balance of proteins and other nutrients that are less reliably provided by diet alone. ONS also eliminate the substantial potential for non-compliance to diet in individuals with
decreased appetite.
Although ONS products differ and are not likely to be interchangeable, innovative formulations provide proteins as well as vitamins and minerals in order to help restore balanced protein metabolism.
A broad nutritional approach is needed because of the interdependence of digestive and metabolic systems. One example of differences between products is the addition of short-chain fructo-oligosaccharides to nourish resident gut bacteria. This addresses the critical role of GI function in absorbing nutrients and maximizing benefits.
In many hospitals or other treatment settings, the greatest obstacle to effective diagnosis and management of PEM is the absence of an advocate or a caregiver designated to perform this function. While dietitians are often available for consults, a systematic approach to all patients is needed. This effort should be led by individuals responsible for daily clinical care, including physicians or nurses. Developing programs with clear responsibilities for nutrition screening would be expected to produce broad benefits and, most importantly,
improved patient outcomes.
Summary
Malnutrition has long been associated with poor outcomes, but there is an increasing body of evidence linking screening and nutrition interventions with large clinical benefits, including reductions in disease complications and in mortality. There are also data demonstrating that the treatment of malnutrition, including the use of ONS, can reduce the risk of these adverse outcomes. From the health care setting perspective, a systematic approach to treatment of malnutrition may reduce costs. From the perspective of patients and caregivers, detecting and treating malnutrition represents an evidence-based strategy to improve outcomes.
References
1. Wolfe RR. The underappreciated role of muscle in health and disease. Am J Clin Nutr 2006;84(3):475-82.
2. Janssen et al. Skeletal muscle mass and distribution in 468 men and women aged 18-88 yr. J Appl Physiol 2000;89(1):81-8.
3. Cereda et al. Body mass index and mortality in institutionalized elderly. J Am Med Dir Assoc 2011;12(3):174-8.
4. Sorensen et al. EuroOOPS: an international, multicentre study to implement nutritional risk screening and evaluate clinical outcome.
Clin Nutr 2008;27(3):340-9.
5. Lammes E, Akner G. Repeated assessment of energy and nutrient intake in 52 nursing home residents. J Nutr Health Aging 2006;10(3):222-30.
6. Norman et al. Prognostic impact of disease-related malnutrition. Clin Nutr 2008;27(1):5-15.
7. Kruizenga et al. Screening of nutritional status in The Netherlands. Clin Nutr 2003;22(2):147-52.
8. Argiles JM. Cancer-associated malnutrition. Eur J Oncol Nurs 2005;9 (Suppl 2):S39-S50.
9. Engelen et al. Nutritional depletion in relation to respiratory and peripheral skeletal muscle function in out-patients with COPD. Eur Respir J 1994;7(10):1793-7.
10. Mijan-de-la-Torre A. Recent insights on chronic heart failure, cachexia and nutrition. Curr Opin Clin Nutr Metab Care 2009;12(3):251-7.
11. Pablo AM, Izaga MA, Alday LA. Assessment of nutritional status on hospital admission: nutritional scores. Eur J Clin Nutr 2003;57(7):824-31.
12. Norman et al. Three month intervention with protein and energy rich supplements improve muscle function and quality of life in malnourished patients with non-neoplastic gastrointestinal disease—a randomized controlled trial. Clin Nutr 2008;27(1):48-56.
13. Collins et al. The impact of malnutrition on hospitalization and mortality in outpatients with chronic obstructive pulmonary disease. Proc Nutr Soc 2010;69:E148 (Abstract).
14. Norman et al. Malnutrition affects quality of life in gastroenterology patients. World J Gastroenterol 2006;12(21):3380-5.
15. Stratton et al. ‘Malnutrition Universal Screening Tool’ predicts mortality and length of hospital stay in acutely ill elderly. Br J Nutr 2006;95(2):325-30.
16. Morley JE, Anker SD, Evans WJ. Cachexia and aging: an update based on the Fourth International Cachexia Meeting. J Nutr Health Aging 2009;13(1):47-55.
17. Cansado P, Ravasco P, Camilo M. A longitudinal study of hospital undernutrition in the elderly: comparison of four validated methods. J Nutr Health Aging 2009;13(2):159-64.
18. Kaiser et al. Frequency of malnutrition in older adults: a multinational perspective using the mini nutritional assessment. J Am Geriatr Soc 2010;58(9):1734-8.
19. Demling RH. Nutrition, anabolism, and the wound healing process: an overview. Eplasty 2009;9:e9.
20. Schneider et al. Malnutrition is an independent factor associated with nosocomial infections. Br J Nutr 2004;92(1):105-11.
21. Humphreys et al. Muscle strength as a predictor of loss of functional status in hospitalized patients. Nutrition 2002;18(7-8):616-20.
22. Pizzigalli et al. Prevention of falling risk in elderly people: the relevance of muscular strength and symmetry of lower limbs in postural stability. J Strength Cond Res 2011;25(2):567-74.
23. Vetta et al. The impact of malnutrition on the quality of life in the elderly. Clin Nutr 1999;18(5):259-67.
24. NICE. Nutrition support in adults: oral nutrition support, enteral tube feeding, and parenteral nutrition. National Institute for Health and Clinical Excellence (NICE) 2006; Clinical Guidelines 32.
25. Stratton RJ. Disease-realted malnutrition: an evidence-based approach to treatment. Wallingford: CABI Publishing; 2003.
26. Milne et al. Protein and energy supplementation in elderly people at risk from malnutrition. Cochrane Database Syst Rev 2005;(2):CD003288.
27. Gariballa et al. A randomized, double-blind, placebo-controlled trial of nutritional supplementation during acute illness. Am J Med
2006;119(8):693-9.
28. Langer et al. Nutritional interventions for preventing and treating pressure ulcers. Cochrane Database Syst Rev 2003(4):CD003216.
29. Stratton RJ, Elia M. Who benefits from nutritional support: what is the evidence? Eur J Gastroenterol Hepatol 2007;19(5):353-8.
30. Elia M, Stratton RJ. A cost-benefit analysis of oral nutritional supplements in preventing pressure ulcers in hospital. Clin Nutr 2005;24:640-1.
31. Rypkema et al. Cost-effectiveness of an interdisciplinary intervention in geriatric inpatients to prevent malnutrition. J Nutr Health Aging 2004;8(2):122-7.
32. Arnaud-Battandier et al. Use of oral supplements in malnourished elderly patients living in the community: a pharmaco-economic study. Clin Nutr 2004;23(5):1096-103.
33. Norman et al. Cost-effectiveness of a 3-month intervention with oral nutritional supplements in disease-related malnutrition: a randomised controlled pilot study. Eur J Clin Nutr 2011;65(6):735-42.
34. Kondrup et al. Incidence of nutritional risk and causes of inadequate nutritional care in hospitals. Clin Nutr 2002;21(6):461-8.
35. Stratton et al. Malnutrition in hospital outpatients and inpatients: prevalence, concurrent validity and ease of use of the ‘malnutrition universal screening tool’ (‘MUST’) for adults. Br J Nutr 2004;92(5):799-808.
36. Kondrup et al. ESPEN Guidelines for Nutrition Screening 2002. Clin Nutr 2003;22(4):415–21.