Reports
“Could Anything Be Worse than COVID?” A Flu Pandemic, Say Influenza Researchers
This report is based on medical evidence presented at sanctioned medical congress, from peer reviewed literature or opinion provided by a qualified healthcare practitioner. The consumption of the information contained within this report is intended for qualified Canadian healthcare practitioners only.
MEDICAL FRONTIERS - Canadian Immunization Conference (CIC 2021)
Online / December 8–9, 2021
Online – While acknowledging that the current focus is on SARS-CoV-2 vaccination, influenza researchers at this year’s Canadian Immunization Conference reminded delegates that, if scaled up to 2021, the 1918–1920 flu pandemic would have killed at least 70 times the number of people than has COVID. They issued a plea to continue to pay attention to new vaccine technology for all pandemic-potential viruses, including flu. In seasonal influenza, the research effort remains directed towards better protection for children and seniors. The conference also heard about early efforts with mRNA vaccines that are tackling the challenges of antigen design in the face of flu’s incredible diversity.
Chief Medical Editor: Dr. Léna Coïc, Montréal, Quebec
Dr. Matthew Miller of the Michael G. DeGroote Institute for Infectious Disease Research, McMaster University, reminded his audience at the CIC that, had the 1918–1920 flu pandemic been scaled to the present world population, 350 million people would have died versus 5 million for COVID to date. “When thinking, could things get any worse? They certainly could,” Dr. Miller said.
He added, “This really underscores the importance of thinking fundamentally differently about how we develop vaccines for viruses that have the risk of causing pandemics.”
Influenza is a particularly terrifying pandemic threat, said Dr. Miller, at a Seqirus-sponsored symposium, because it’s a moving target: “The tremendous diversity of influenza viruses…is what challenges our ability to make vaccines that are broadly protective – there are just so many ‘flavours’ of the virus out there in nature – and we have ostensibly no ability to predict which may be a pandemic risk in the future.”
Introducing a plenary on mRNA technology for flu vaccination, Dr. Guillaume Poliquin, acting Vice-President of Canada’s National Microbiology Laboratory, said we’re effectively relying on our “best guess” to guide the sophisticated and complex machinery that is seasonal flu vaccine production, despite the vigilance of over 140 global-surveillance laboratories that funnel flu-strain information into six WHO collaborating centres. “That feeds into the need for innovation,” Dr. Poliquin concluded.
Referring to the “impressive array” of vaccines now available in Canada, Dr. Miller pointed out that “A lot of the recent innovation in the seasonal-flu vaccine field has been driven by a desire to provide better protection to special populations.”
Influenza-Vaccine Innovations
Focused on Children
Sometimes innovation is simply a matter of changing targets. For example, despite the fact that infants under 6 months are at high risk for flu mortality and morbidity, there is currently no vaccine in Canada licensed for babies of this age. One solution is to vaccinate their mothers during pregnancy.
Dr. Deshayne Fell, associate professor of epidemiology at the University of Ottawa, analyzed the effectiveness of this approach in Ontario over nine flu seasons (2010–2011 to 2018–2019).1 She and her colleagues linked laboratory flu-test results with maternal flu-vaccination status in 23,806 babies. Vaccine effectiveness (VE) was calculated by comparing the odds of a ‘test-positive’ baby having a mother vaccinated during pregnancy to the same odds for a ‘test-negative’ baby.
By this measure, overall VE of maternal flu vaccination against lab-confirmed flu in the newborn infant in Ontario was 64%. Fell noted that this VE was the highest seen for similar studies. [See Q&A.]
A recent approach to improve flu VE for children is cell-based vaccines, instead of using avian eggs as a growth medium. This eliminates the risk of last-minute egg-adaptive mutations that lead to an antigen mismatch with the WHO-selected strain that season, explained Dr. Sherilyn Houle, an assistant professor at the School of Pharmacy, University of Waterloo, Ontario. Dr. Houle, speaking during a Sanofi-Pasteur-sponsored event, reviewed the specs for Canada’s first and only cell-based flu vaccine (QIVc; Flucelvax Quad), approved for individuals over 2 years old.
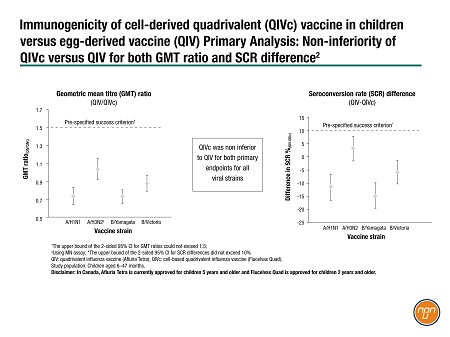
Dr. Marten Heeringa of Seqirus presented new phase-3 data comparing cell-derived Flucelvax Quad with an egg-derived vaccine (QIV; Afluria Tetra) in children 6–47 months of age.2 This trial was the first test of a cell-based vaccine in children 6–23 months old. [In Canada, Flucelvax Quad is not approved in children aged 6–23 months and Afluria Tetra is not approved in children under 5 years.]
The trial took place during the 2019–2020 northern hemisphere flu season at 47 U.S. centres. Children were randomized 2:1 to QIVc:QIV. The primary endpoints were non-inferiority for geometric mean titre (GMT) ratios and for seroconversion rates (SCR). The study found that QIVc was non-inferior to QIV for both primary endpoints for all viral strains (Figure 1).
Figure 1.
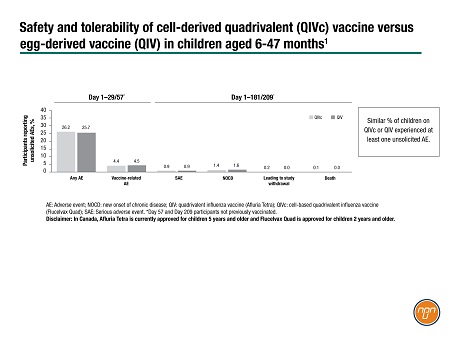
During the trial, a similar percentage of children experienced at least one unsolicited adverse event (AE) on the two vaccines, most commonly upper-respiratory-tract infection or pyrexia (Figure 2). The most common vaccine-related unsolicited AEs were injection-site bruising and irritability. The two fatal serious adverse events occurred among the children who received QIVc: an adenoviral encephalopathy deemed unrelated to the vaccine on adjudication and a traffic accident.
Figure 2.
Influenza-Vaccine Innovations
Focused on Older Adults
“We have this idea that perhaps immune function is just lower in older adults, but in fact, it's not always lower, it's more dysregulated,” said Dr. Melissa Andrew, Division of Geriatric Medicine, Dalhousie University, Nova Scotia. Dr. Andrew was speaking during a Sanofi-Pasteur-sponsored event.
She said the focus should be, not chronological age, but frailty – since it’s frailty that affects flu-vaccine effectiveness. Frailty also increases flu mortality, according to data Dr. Andrew presented: flu mortality was 23.5% (38/162) for moderate-severe frailty versus only 3.2% (12/376) in non-frail older adults.3
A frail patient may also permanently lose function after a bout of flu; this loss of function is “critical to understanding the true burden of influenza,” Dr. Andrew said. Frailty is also dangerous because standard influenza-like illness (ILI) and severe-acute respiratory infection (SARI) criteria may not apply.4 “More than half the patients admitted to hospital…are missed if we wait for them to have a fever or cough,” said Dr. Andrew.
Dr. Houle of the University of Waterloo reminded her audience that there are two vaccines indicated for adults ≥ 65 years old in Canada: a new quadrivalent high-dose flu vaccine (IIV4-HD, Fluzone HD Quadrivalent) and an adjuvanted vaccine (IIV3-adj, Fluad).
Due to the lack of head-to-head data on IIV4-HD and IIV3-adj, NACI has concluded that there was “insufficient evidence” to recommend one over the other.
Several speakers commented on the difficulties of conducting head-to-head vaccine trials in a disease like flu, which is an immunological moving target each year. Dr. Miller suggested that real-world evidence (RWE) can overcome this challenge if the datasets are large enough. He presented a retrospective analysis published in September, 20215, of linked EMR and claims data in approximately 11 million individuals aged ≥ 65 in the U.S.
In the RWE study, adjuvanted vaccine was significantly more effective than the high-dose vaccine in both the 2017–2018 and 2018–2019 flu seasons (adjusted rVE 3.6 [CI 1.4–5.7] and 7.4 [CI:2.3–12.8], respectively).
mRNA Flu Vaccines
“One of the few silver linings of the pandemic – they're few and far between – has been the ability to push the envelope in terms of vaccine technologies,” said Dr. Poliquin of the NML in the plenary on mRNA technology for influenza.
Dr. Anna Blakney, assistant professor, University of BC, Vancouver, BC, recalled during her plenary presentation how quickly her former team at Imperial College, London, U.K., had been able to create an mRNA vaccine against SARS-CoV-2. Dr. Blakney said that the scientists received the sequence of the circulating strain on January 10, 2020, and went to clinical trials on June 19: “And this was an academic team [not a commercial one],” she commented.
Apart from production speed, another advantage of mRNA vaccines is the small manufacturing volumes because they are synthesized in a bioreactor, said Dr. Blakney: “You can make about a million doses in 100 mL volume.” Dr. Blakney calculated that two Olympic-sized swimming pools-worth of vaccine could supply the entire world.
“That’s a total game-changer for RNA vaccines,” she said. “So you’re probably asking…why don’t we have an mRNA flu vaccine yet?” Dr. Blakney said that, despite the advantages of the technology and at least four companies with “candidates currently in the clinic,” flu vaccines are still “an uphill battle”. The challenges are three-pronged: optimizing the dose and immunogenicity (an early Moderna mRNA flu vaccine worked well in animals but not in humans) and antigen design.
“Influenza viruses are incredibly diverse…there are over 18 known subtypes of hemagglutinin in nature and at least 11 known subtypes of neuraminidase [for influenza A],” said Dr. Miller.
“Biology is tricky,” concluded Dr. Poliquin. “Influenza, in particular, is a frustrating pathogen. So human ingenuity will need to battle with Mother Nature's infinite wisdom, it seems, and hopefully will be victorious – but it will be an ongoing process.”
Conclusions
Influenza sessions at this year’s CIC reminded delegates that influenza as a pandemic threat has not retreated and, despite the focus on COVID-19, efforts to innovate in flu vaccination should continue. The focus of innovation to date has been vulnerable populations such as young children and frail, older adults. With now-proven vaccine technologies such as mRNA in the pipeline, the focus is shifting to flu vaccines more robust in the face of seasonal or pandemic antigenic shifts.
Questions and Answers
Questions and answers on work presented at CIC 2021–CCI 2021. MedNet invited commentary from Dr. Rupesh Chawla, Associate Professor, Pediatric Infectious Diseases, University of Saskatchewan, Saskatoon, Saskatchewan.
Q: How do COVID-19 and influenza compare in terms of risks for young children?
Dr. Chawla: This age group is not a high-risk group at all for COVID-19; as we know, the risk has been very low. By contrast, young kids are high-risk for influenza; especially under the age of two, where we see a much higher risk for mortality and morbidity.
Q. What learnings from childhood influenza are being applied to the management of SARS-CoV-2 in children?
Dr. Chawla: It's general supportive management [for both diseases]. They tend to get more bronchiolytic with
SARS-CoV-2 but they don't tend to get as sick as with the influenza, where they tend to end up in intensive care, intubated and quite unwell with secondary bacterial pneumonias at much higher rates.
Q: The study by Fell and colleagues at CIC found that maternal flu vaccination had a 64% VE against influenza infection in their babies in the first 6 months. What is happening immunologically?
Dr. Chawla: We know that there's not a good immune response that can be produced by an infant, especially under the age of six months, so the real thought process has been, how do we provide that immunity in a more effective way? And we know the placental transfer of antibodies is a really important way to provide antibodies and sometimes they can last for up to
18 months – it’s amazing. So the best way to give them that immunity is to actually immunize the mother, not the infant.
Q: The study also found that only 9.1% of babies in Ontario were born to people vaccinated against flu in pregnancy. What are the clinical implications of this low uptake and what should medical professionals do about it?
Dr. Chawla: I'm glad they looked at it. We’ve always known that pregnant women are a high-risk group so I was surprised the vaccination rate was that low in Ontario. In Alberta [where I come from], the obstetrician-gynecologists are rabid about promoting influenza vaccination for their pregnant patients. People think about the very old and the very young being at risk, but forget that very important group: pregnant patients who are at very high risk of getting sick.
Q: How would you advise healthcare professionals to counsel a pregnant person hesitant to be vaccinated for flu?
Dr. Chawla: That’s part of our role, as educators in terms of influenza, to remind people that pregnancy is a high-risk time. And not only are you getting that benefit for the mum, but now, with this sort of data, you can say that you're really not just providing for mum, but for her child. I mean, how better to say it than this: “Not only are you at risk, but you're giving your baby the best chance”?
Q: What advances in the flu vaccine field would you like to see, especially for children?
Dr. Chawla: We certainly need a better assay to determine how well a vaccine is working. But it’s an exciting world right now. We've always been searching for that what we call the ‘golden vaccine’, the universal flu vaccine that doesn't require regular boosting, or at least not yearly boosting. But the problem is, we’re basing vaccine development on hemagglutinin and neuraminidase, which do change. So if we could use a different portion of the virus, you might have a more universal vaccine.
References:
1. Fell D. Effectiveness of influenza vaccination during pregnancy on laboratory-confirmed seasonal influenza among infants under 6 months of age in Ontario. Data presented at CIC 2021–CCI 2021.
2. Heeringa M. Immunogenicity and safety of cell-derived quadrivalent influenza vaccine in children 6 through 47 months: A randomized controlled non-inferiority trial. Data presented at CIC 2021–CCI 2021.
3. Andrew M. Protection beyond flu: what’s new for influenza vaccines among adults 60+. Data presented at CIC 2021–CCI 2021.
4. Andrew MK, McElhaney JE, McGeer AA, et al. Influenza surveillance case definitions miss a substantial proportion of older adults hospitalized with laboratory-confirmed influenza: A report from the Canadian Immunization Research Network (CIRN) Serious Outcomes Surveillance (SOS) Network. Infect Control Hosp Epidemiol 2020; 41:499–504.
5. Boikos C, Fischer L, O’Brien D, et al. Relative effectiveness of adjuvanted trivalent inactivated influenza vaccine versus egg-derived quadrivalent inactivated influenza vaccines and high-dose trivalent influenza vaccine in preventing influenza-related medical encounters in US adults ≥ 65 years during the 2017–2018 and 2018–2019 influenza seasons. ClD 2021; 73:816–823.