Reports
Direct-acting Antiviral Agents in Chronic HCV Therapy: Exploring Less Complex Regimens
This report is based on medical evidence presented at sanctioned medical congress, from peer reviewed literature or opinion provided by a qualified healthcare practitioner. The consumption of the information contained within this report is intended for qualified Canadian healthcare practitioners only.
MEDICAL FRONTIERS - 63rd Annual Meeting of the American Association for the Study of Liver Diseases
Boston, Massachusetts / November 9-13, 2012
Boston - Therapy for chronic hepatitis C viral infections has changed significantly, thanks to the development of direct-acting antiviral agents that target specific vulnerabilities of the virus in all or most of its variant forms. Additionally, expansion of investigational agents used in combinations offer the promise of significantly higher sustained viral response rates and efficacy in previously hard-to-treat patients with therapy-resistant genotypes or viral mutations. The 2012 edition of the Liver Meeting brought news of both investigational antiviral agents in the laboratory and in clinical trials, and of welcome clinical advances that make the lives of patients with chronic HCV infections easier, and give clinicians greater assurance that their patients will be more likely to adhere to the highly effective regimens they prescribe.
Chief Medical Editor: Dr. Léna Coïc, Montréal, Quebec
The advent of direct-acting antiviral agents (DAAs), notably the NS3/4A protease inhibitors boceprevir and telaprevir, has transformed the face of therapy for patients with chronic hepatitis C (HCV) viral infections. Paired with pegylated interferon alpha (PEG-IFN) and ribavirin (RBV) therapy, these agents have dramatically and rapidly reduced HCV RNA levels in a majority of patients, and are associated with sustained virologic response (SVR) rates that many equate with a cure.
However, compliance with treatment regimens that may last 24 to 48 weeks and require thrice-daily dosing with specific food pairings can be challenging for many patients. Fortunately, as seen over the last decade with therapies for HIV/AIDS, therapeutic regimens for chronic HCV infections are moving toward less complex, more convenient regimens without sacrificing efficacy or safety. Indeed, several investigational regimens appear to provide good efficacy and tolerability without the need for PEG-IFN, which is associated with significant toxicities, and in some cases without RBV, which is associated with hematologic adverse events.
Convenience in Dosing Regimens
One example of the move toward more convenient dosing regimens can be seen in data from the large multicentre OPTIMIZE Trial (A Randomized, Open-label, Phase 3 Study of Telaprevir Administered Twice Daily or Every 8 Hours in Combination With Pegylated Interferon Alfa-2a and Ribavirin in Treatment-naive Subjects With Genotype 1 Chronic Hepatitis C Virus Infection).
Dr. Maria Buti, Professor of Medicine and Chief of Internal Medicine and Hepatology, Hospital Universitari Vall d’Hebron, Barcelona, Spain, and colleagues, compared 2 telaprevir dosing strategies in 730 treatment-naive patients with HCV genotype 1 infections: 750 mg every 8 hours (q8h) or 1125 mg b.i.d., in combination with PEG-IFN/RBV at standard doses for 12 weeks.
At the end of 12 weeks of combined therapy, patients in both trial arms were continued on PEG-IFN/RBV. Those patients who achieved a rapid viral response (RVR), defined as HCV RNA <25 IU/mL (target not detected) at week 4 of treatment, were treated for a total of 24 weeks. All other patients received a total of 48 weeks of therapy.
Patients were monitored to ensure that resistance mutations did not emerge and to certify that patients were being treated in accordance with chronic HCV genotype 1 infections standard guidelines. If patients had HCV RNA levels >1000 IU/mL at week 4 of treatment or if they were ≥25 IU/mL at weeks 12, 24, 32 or 40, all study drugs would be stopped. The investigators defined on-treatment virologic failure as viral breakthrough or meeting the pre-specified virologic stopping rule.
Non-inferiority of Twice-daily Dosing
The trial met its primary objective of non-inferiority (Figure 1). The SVR rates 12 weeks after the end of treatment (SVR12) were 74% in the b.i.d. group and 73% in the q8h group (difference 1.5%, 95% CI, -4.9% +12.0%). Non-inferiority was established by the fact that the lower range of the confidence interval, -4.9%, was significantly higher than the predetermined non-inferiority margin of -11%, the authors reported in a late-breaking poster presentation.
Figure 1.
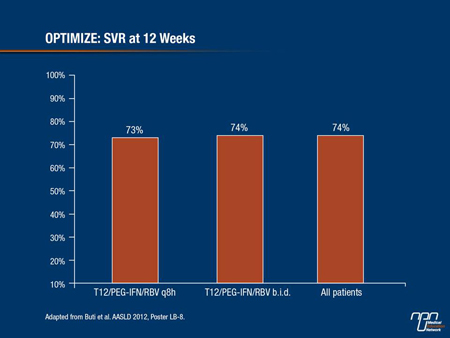
Subgroup analyses also showed that the 2 dosing regimens had similar activity in patients with cirrhotic disease (SVR12 rates of 54% for b.i.d. dosing and 49% for q8h), and non-cirrhotic disease (SVR12 78% and 77%, respectively).
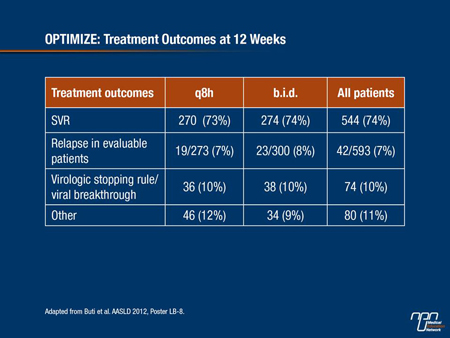
As noted before, patients with RVR at 4 weeks were eligible for 24-week rather than 48 week therapy; about two-thirds of patients met the criteria for shorter duration therapy. Among patients with RVR at week 4, 86% of those on the b.i.d. dose had an SVR, compared with 85% in the q8h group. Among patients negative for RVR at week 4, SVR rates were 47% in each group.
Relapse rates were also similar between the dosing groups, at 8% among those in the b.i.d. group and 7% among patients in the q8h group. Serious adverse events occurred in 35 of 371 patients (9%) assigned to b.i.d. dosing, and in 28 of 369 (8%) assigned to q8h. One on-study death (brain neoplasm) was ruled not related to either telaprevir or PEG-IFN/RBV in the b.i.d. group by the investigator.
Adverse events of grade ≥3 occurred in 38% and 42% of patients on q8h and b.i.d. regimens, respectively. Grade 4 events occurred in 7% and 6% of patients, respectively.
Table 1:
Canadian Experience
In Alberta, third-party approval by the provincial payers was just obtained for telaprevir, noted Dr. Robert Myers, Assistant Professor, Liver Unit, University of Calgary, Alberta, and the lead author of Canadian consensus guidelines on the management of chronic HCV.
The availability of the DAAs throughout Canada is making a substantial difference in clinical practice, remarked Dr. Myers. “It’s early days in terms of evaluating treatment responses, because of the short time-frame that they have been available in Canada, but based on the clinical trial data, they look quite promising,” he noted. “In patients with genotype 1, treatment-naive and treatment-experienced patients, telaprevir and boceprevir are definitely first-line therapy now.”
The currently approved q8h dosing schedule of these agents is a large hurdle to overcome, as is the relatively large pill burden, which is why the OPTIMIZE results represent “a significant improvement that once approved by the regulatory agencies will make managing these patients much easier,” Dr. Myers emphasized.
OPTIMIZE Dose Regimens
Since RBV is already delivered in b.i.d. oral doses, telaprevir b.i.d. would simplify the drug regimen for most patients, and increase the likelihood of compliance. “Being able to go from 3 times a day to 2 times a day doesn’t sound like a big deal, but this will be very useful for patients,” he said.
Dr. Stephen Shafran, Professor of Medicine, Division of Infectious Diseases, University of Alberta, Edmonton, said that “OPTIMIZE is a definite step forward, because t.i.d. dosing is logistically difficult for people.”
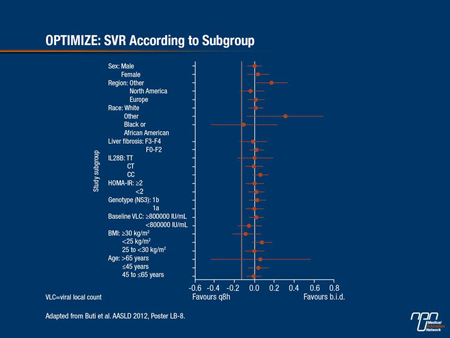
He noted that in the randomized controlled trial, “Patients were very well balanced for characteristics, and when you start slicing and dicing subpopulations, there was really no subpopulation in which a problem with b.i.d. dosing appeared (Figure 2). I think patients are going to be way more adherent to a b.i.d regimen. It’s a definite advantage - I don’t think one can call it a ‘game changer,’ but it’s certainly going to make life easier.”
Figure 2.
HIV/HCV Co-infection
The problem of finding the most effective therapeutic regimen for HCV infection becomes magnified among patients co-infected with HIV. Such co-infected patients are at greater risk for developing cirrhosis, end-stage liver disease, hepatocellular carcinoma and for death from liver disease, noted Dr. Mark Sulkowski, Director, Viral Hepatitis Center, Johns Hopkins University of Medicine, Baltimore, Maryland.
Although HIV can be effectively controlled in a majority of patients with highly active anti-retroviral therapy (HAART) and HCV can be effectively eradicated with DAAs, there has been concern about safety on adding a DAA plus PEG-IFN/RBV to a HAART regimen, and whether efficacy of the respective regimens could be compromised by drug-drug interactions.
Results from a phase III randomized, double-blind, placebo controlled trial (Study 110), in which patients received a specific combination of anti-retroviral agents (ART) with PEG-IFN/RBV ±telaprevir was reported.
The trial had 2 parts: in part A, patients were with HIV/HCV co-infection were assigned on a 1:1 ratio to receive either telaprevir or placebo, each with PEG-IFN/RBV. In part B patients on an ART (either a combination of efavirenz, tenofovir and emtricitabine or ritonavir-boosted atazanavir, tenofovir and emtricitabine or lamivudine) were assigned on a 2:1 basis to either telaprevir or placebo plus PEG-IFN/RBV. All patients were treated for 48 weeks and followed for an additional 24 weeks.
The investigators measured HCV RNA levels at various time points and looked for drug interactions, adverse events (AEs), viral breakthrough and other clinical measures.
In part A, the SVR at post-treatment week 24 (SVR24) among telaprevir-treated patients was 71% compared with 33% among those treated with placebo.
In the part B cohorts, 80% achieved an SVR24 for those receiving telaprevir in the atazanavir-containing regimen compared with 50% of controls without telaprevir. In the efavirenz-containing regimen the SVR24 was 69% compared to 50%, respectively.
Importantly, the safety profile of the regimens with added telaprevir was similar to that seen without the DAA, and the serum concentrations of both efavirenz and atazanavir were similar with or without telaprevir. There were no HIV viral breakthroughs and although CD4 cell counts declined in patients taking both telaprevir or placebo and PEG-IFN/RBV, there were no HIV-related AEs, Dr. Sulkowski remarked.
Although a telaprevir dose increase was required among patients also taking efavirenz, the data show that “the dose increase of telaprevir in the context of efavirenz appeared to be effective in maintaining exposure to this agent,” he said.
“Overall, 74% of patients treated with telaprevir in combination with PEG-IFN/RBV achieved SVR compared to 45% of those treated with placebo control. Drug interactions with telaprevir and selected anti-retroviral therapies, specifically atazanavir/ritonavir and efavirenz, were not clinically meaningful,” Dr. Sulkowski emphasized.
IFN-free Regimens: A Possible Future
Several oral and poster presentations at the 2012 Liver Meeting focused on various combinations of IFN-free regimens containing investigational DAAs in varying combinations, including one once-daily drug in combination with RBV.
Dr. Gregory T. Everson, Professor of medicine, University of Colorado School of Medicine, Denver and colleagues, reported that a triple DAA regimen combining daclatasvir, an investigational viral NS5A replication complex inhibitor, asunaprevir, an investigational NS3 protease inhibitor and BMS-791325, a non-nucleoside NS5B polymerase inhibitor yielded high SVR rates after both 12 and 24 weeks of treatment in previously untreated, non-cirrhotic patients with HCV genotype 1 chronic infections.
Dr. Sulkowski and colleagues reported on a combination of daclatasvir and the HCV polymerase inhibitor sofosbuvir ±RBV, in patients with HCV genotypes 1, 2 and 3 treated for 24 weeks. They found that the combinations achieved SVR in more than 93% of the entire patient sample and that among 126 patients with genotype 1, 96% of those who had reached 12 weeks post-treatment had an SVR12, including 3 who did not have SVR4.
In the ELECTRON study, Prof. Edward John Gane, Professor of Medicine, University of Auckland, New Zealand, and colleagues, tested whether regimens containing the nucleotide analog sofosbuvir as a backbone ±PEG-IFN and/or RBV or even as a stand-alone agent could be effective in treatment-naive and treatment-experienced (null responder) patients.
They found that in HCV genotype 2 or 3 infections (more common outside of Canada and the US, where genotype 1 infections predominate), sofosbuvir plus RBV for 12 weeks was safe and effective in both treatment-naive and experienced patients, but that shorter duration regimens or reduced RBV dose could adversely affect efficacy.
They also found that for genotype 1, sofosbuvir and RBV appeared to need help from a third agent, GS-5885, an inhibitor of the NS5A polymerase, in order to knock down infections in treatment-naive patients. Sofosbuvir and RBV alone produced SVR in only 10% of null responders, they reported.
In a separate study, Dr. Anu Osinusi, Clinical investigator, US National Institute of Allergy and Infectious Diseases, Bethesda, Maryland, and colleagues, reported on a study comparing sofosbuvir with either full- or low-dose RBV in an urban population. In an intention-to-treat analysis, full dose RBV achieved an SVR4 of 72% and low-dose 64% (modified ITT including a patient who left the study early but was later followed). Both regimens were well tolerated and both produced significant improvement of inflammation with treatment, Dr. Osinusi said.
Summary
It is clear that therapy for chronic HCV is rapidly evolving and improving, and according to Dr. Raymond T. Chung, Director of Hepatology, Massachusetts General Hospital, Boston, “prospects for all-oral, tolerable, high efficacy regimens are excellent.”
Investigational therapies with high barriers to viral resistance offer the promise of simpler regimens. RBV at present is important for achieving freedom from IFN in less-than-optimal regimens but the use of combination DAA regimens might provide a door to IFN-free regimens. The addition of DAAs to HCV therapy is moving forward to a more individualized approach.